INTRODUCTION
Citrus is a major fruit crop grown worldwide, with oranges, tangerines, lemons, citron, and grapefruits accounting for 62, 17, 11, and 10% of the total citrus production in 2020, respectively [FAO, 2021]. The consumption of citrus fruits, both fresh and processed by the food industry, generates more than 54 million tons of waste annually on a global scale, with 50–60% of these unused by-products consisting mainly of peels [Teigiserova et al., 2021]. Citrus fruit peels are a rich source of dietary fiber and phenolic compounds, including flavonoids, which contribute to their natural antioxidant activity [Ani & Abel, 2018; Makni et al., 2018]. Among citrus by-products, Citrus sinensis (orange) peel contains 46.5% of total dietary fiber, with soluble dietary fiber (SDF) and insoluble dietary fiber (IDF) fractions accounting for 9.2% and 37.3% of peel, respectively [Núñez-Gómez et al., 2024]. However, the structural characteristics of dietary fiber extracted from orange peel are largely influenced by the composition of its cell wall polysaccharides, such as pectin, cellulose, hemicellulose, and lignin [de Castro et al., 2024]. Both dietary fiber fractions offer distinct health benefits, such as improved digestive health, cholesterol reduction, and glycemic control [Dhingra et al., 2012]. In addition to these health benefits, dietary fiber possesses desired functional properties, such as water retention, oil retention, and gel formation, making it a valuable industrial food additive for improving nutritional content and texture [Elleuch et al., 2011; Tejada-Ortigoza et al., 2016].
The extraction method significantly influences the structural and functional properties of the resulting dietary fiber. Acid, alkaline, and enzyme-assisted extractions, modify fiber composition and physicochemical properties, enhancing their potential applications in food formulations [Berktas & Cam, 2025; Tang et al., 2024; Zhang et al., 2017]. These variations are further affected by the nature of the raw material and the extraction conditions used, which ultimately determine the molecular structure, surface morphology, and degree of polymerization of the extracted SDF [Berktas & Cam, 2025; Wang et al., 2015]. For instance, treating IDF through mild acid hydrolysis (e.g., 0.2% sulfuric acid) can convert part of it into soluble components, thereby enhancing water-holding capacity (WHC) and water solubility (WSC) by removing starch and protein, along with microstructural changes [Qi et al., 2015]. However, the efficiency of chemical extraction methods depends heavily on the food matrix and nature of the extracted polysaccharides [Wang et al., 2022]. For example, fibers extracted from sugar beet have much higher WHC and WSC values than those from pea hulls when alkaline extraction methods are used [Bertin et al., 1988; Weightman et al., 1995]. Chemical extraction methods, such as acid or base treatment, are relatively simple and cost-effective. They can be applied to a wide range of raw materials, leading to high extraction yields and modifications of fiber structure and properties [Qi et al., 2015; Tejada-Ortigoza et al., 2016]. However, the major drawback of these methods is the potential destruction of food structure due to prolonged processing times and high temperatures. This can lead to the degradation of polysaccharides with functional groups such as hydroxyl and carboxyl groups, which can negatively impact the structural integrity and functional properties of the resulting dietary fiber [Berktas & Cam, 2025; Wang et al., 2015].
To mitigate environmental and operational challenges, such as high temperatures and equipment corrosion, enzyme-assisted extraction has been developed as an alternative. It can increase the SDF/IDF ratio by selectively hydrolyzing insoluble polysaccharide chains (e.g., cellulose and hemicellulose) into smaller, water-soluble fragments [Zhao et al., 2025; Zheng & Li, 2018]. This enzymatic breakdown also exposes more hydrophilic functional groups, such as hydroxyl groups, thereby enhancing water-holding capacity and surface area [Tang et al., 2024; Yoon et al., 2005]. However, enzymatic methods are limited by their complexity, time consumption, high enzyme costs, and the need for precise temperature control [Elleuch et al., 2011]. Despite extensive research on fiber extraction, optimizing the method to maximize yield and functionality while maintaining environmental sustainability remains a challenge. This study evaluated the effects of different extraction methods (acidic, alkaline, and enzyme-assisted) on the yield, structure, and physicochemical properties of SDF preparations from C. sinensis peel, with the aim of identifying the most effective approach for future practical applications in food processing.
MATERIALS AND METHODS
Chemicals and reagents
A dietary fiber analysis kit was obtained from Megazyme (Wicklow, Ireland). All chemicals used in this study, including sodium phosphate dodecahydrate (Na2HPO4×12H2O), sodium dihydrogen phosphate dihydrate (NaH2PO4×2H2O), and citric acid, were purchased from Merck (Darmstadt, Germany). Enzymes used for fiber extraction – xylanase with an activity of 100,000 U/g and cellulase with an activity of 10,000 U/g were purchased from Angel Yeast Co., Ltd (Yichang, Hubei, China). Ethanol used in all pre-treatment steps was sourced domestically from Vietnam.
Material and its preparation
The orange by-products (collected after segment separation or juice extraction) were obtained directly from VinaGreenCo One Member Co., Ltd. (My Hoa Commune, Binh Minh Town, Vinh Long Province, Vietnam). Upon receipt, the outer peels were discarded. The remaining white peels, cores, and pulp were thoroughly rinsed with running water to remove any residual fruit flesh. The components were then chopped and subjected to ethanol pre-treatment by soaking in 96% ethanol at a 1:3 (w/v) ratio for 30 min at 60°C, using a reflux system equipped with a 40-cm condenser and thermometer attached to a two-neck flask. Following ethanol treatment, the samples were rinsed with 70% (v/v) ethanol and dried at 60°C to a constant weight (moisture content approximately 4 g/100 g). The dried material was ground using an SK200 dry mill (SEKA, Tokyo, Japan), and the resulting powder was passed through an 80-mesh sieve to ensure uniform particle size [Huong et al., 2024]. The final orange powder was stored in vacuum-sealed polyamide (PA) bags at room temperature (25°C) until further analyses.
Methods of soluble dietary fiber extraction
Acid extraction
The acid extraction method was adapted from Kermani et al. [2014] and Dong et al. [2020]. Ten grams of orange peel powder were mixed with a 4% citric acid solution at a solid to liquid ratio of 1:40 (g/mL) and heated at 70°C for 90 min, pH 1.9. After extraction, the mixture was centrifuged at 2,800×g for 10 min, and the supernatant was collected. Then, supernatant was mixed with two volumes of 96% ethanol and left undisturbed for 2 h. The resulting precipitate was filtered, and dried at 60°C to a constant weight, yielding soluble dietary fiber preparation obtained under acid conditions, referred to as A-SDF.
Alkaline extraction
The method of extraction under alkaline conditions was adapted from Wang et al. [2015] with slight modifications. Orange peel powder (10 g) was suspended in a 0.5% NaOH solution at a solid to liquid ratio of 1:40 (g/mL) and heated at 60°C for 60 min. After extraction, the mixture was centrifuged at 2,800×g for 10 min, and from the supernatant, the SDF was precipitated by adding two volumes of 96% ethanol and left for 2 h. Then, the precipitate was filtered and dried at 60°C to a constant weight, yielding an SDF preparation obtained under alkaline conditions, denoted as B-SDF.
Enzyme-assisted extraction
Enzyme-assisted extractions of orange peel powder were carried out according to the procedure used previously by Dong et al. [2020]. Orange peel powder (10 g) was mixed with 500 mL of citrate buffer and hydrolyzed with cellulase (enzyme to powder ratio, E/P, of 0.2%, w/w) at 50°C, pH 5, for 60 min, or with xylanase (E/P of 0.2%, w/w) at 60°C, pH 4.5, for 90 min. The enzymes were then inactivated by heating at 90°C for 15 min. SDF was precipitated following a procedure described above for the acid extraction, yielding SDF preparations (EC-SDF and EX-SDF, respectively).
Extraction efficiency calculation
The extraction efficiency (EE, %) of acid, alkaline, and enzymeassisted methods was calculated using Equation (1):
where: M1 is the mass of the SDF preparation obtained after drying (g) and M0 is the mass of the orange peel powder (g).
Determination of fiber composition
The content of IDF and SDF in SDF preparations was determined following AOAC International methods no. 991.42 and 993.19, respectively [AOAC, 2005]. Total dietary fiber was calculated as a sum of SDF and IDF. The powdered SDF preparations were mixed with phosphate buffer and hydrolyzed sequentially with α-amylase (pH 6.0) at 100°C for 15 min, protease (pH 7.5) at 60°C for 30 min, and amyloglucosidase (pH 4.5) at 60°C for 30 min to hydrolyze starch and protein. The resulting residue (IDF) was filtered and washed thoroughly with distilled water, ethanol, and acetone. The filtrate was mixed with 96% ethanol (at a liquid/ethanol ratio of 1:2, v/v) at room temperature (25°C) for 1 h. The resulting precipitate with SDF was further washed with ethanol and acetone to obtain the SDF fraction. Weights of both IDF and SDF residues were corrected for protein (determined as Kjeldahl nitrogen × 6.25) and ash content, as specified in the AOAC protocols. Final dietary fiber contents were calculated on a dry weight basis and expressed as g per 100 g of dry matter of preparation (g/100 g dm).
Determination of pectin content in SDF preparations was performed based on the method described by Carré & Haynes [1922] with slight modifications. The dried SDF preparations were treated with oxalic acid, pH 4.6 (at a solid to liquid ratio of 1:40, w/v) at 85oC for 1 h. The mixture was filtered through a nylon cloth to remove the residues, and pectin was precipitated by adding twice volumes of 96% ethanol. The precipitate was washed several times with 70% ethanol and dried at 50oC. An aliquot of 0.15 g (W) of this crude pectin was neutralized with 100 mL of 0.1 M NaOH. Subsequently, 50 mL of 0.1 M acetic acid was added and allowed to react for 5 min at room temperature (25°C), followed by the addition of 50 mL of 1 M CaCl2. After equilibrating for 1 h at room temperature (25°C), the mixture was boiled for 5 min. The formed precipitate was filtered and washed with hot water until no chloride ions were detected (tested with a 1% AgNO3 solution). The final residue was dried at 105°C and weighed. Pectin content was calculated using Equation (2) and expressed in g per 100 g of dry SDF preparation (g/100 g dm):
where: W1 is the weight of the dried calcium pectate (g), W is the weight of the SDF sample used for precipitation (g), and 0.92 is the conversion factor accounting for calcium content in the precipitate.
Cellulose content was determined according to the method of Liu et al. [2021] with minor modifications. One gram of dried SDF preparation (W) was mixed with 25 mL of a nitric acid–ethanol solution (1:4, v/v). The suspension was refluxed in a boiling water bath for 1 h, filtered, and the insoluble residue was collected. The procedure was repeated until the fibers appeared white. The residue was washed with warm distilled water to neutral pH, followed by two rinses with 96% ethanol, and dried at 105°C to a constant weight (W1). The dried residue was then ashed at 575oC, and the ash weight was recorded (W2). The cellulose content (g/100 g dm) was calculated using Equation (3):
Hemicellulose content in SDF preparations was determined by subtracting the cellulose content from the holocellulose content. The latter was determined by the method previously used by Viera et al. [2007]. One gram of dried SDF preparation (W) was combined with 0.75 g of sodium chlorite, 0.5 mL of glacial acetic acid, and 100 mL of distilled water in a 250-mL beaker. The mixture was stirred until the chlorite dissolved, then incubated in a water bath at 70°C for 1 h with occasional stirring. The same amounts of reagents were added every hour over the next two hours, resulting in a total digestion time of 3 h. The mixture was cooled to 10°C, filtered, and washed six times with ice-cold water. The resulting holocellulose was dried at 105°C for 6 h, and weighed (W1). The cellulose content determined previously was recorded as W2. The hemicellulose content (g/100 g dm) was calculated using Equation (4):
Determination of functional properties
Water solubility
The water solubility (WS) of SDF preparations was determined using the method described by Wang et al. [2015], with slight modifications. One gram of dried SDF sample (W) was mixed with distilled water (5 mL) in a 15-mL centrifuge tube. The mixture was heated at 90°C for 30 min in a water bath, mixed every 5 min for 30 s, and centrifuged at 2,800×g for 10 min. The supernatant was collected, dried at 105°C, and weighed (W1). The WS (g/g) was calculated using Equation (5):
Water holding capacity
The water holding capacity (WHC) was determined following the method described by Dong et al. [2020], with minor modifications. The dried SDF preparation (W, 0.5 g) was mixed with 20 mL of distilled water in a 50-mL centrifuge tube. After equilibration at room temperature (25°C) for 24 h, the sample was centrifuged at 2,800×g for 10 min. The supernatant was discarded, and the weight of the hydrated residue (W1) was recorded. The WHC (g/g) was calculated using Equation (6):
Oil holding capacity
The oil holding capacity (OHC) was determined according to the method described by Sangnark & Noomhorm [2003] with minor modifications. The dried SDF preparation (W, 0.5 g) was mixed with soybean oil (5 mL) in a 15-mL centrifuge tube. After equilibration at 4°C for 1 h, the sample was centrifuged at 2,800×g for 20 min. The unbound oil was discarded, and the weight of the oil-retained residue (W1) was recorded. The OHC (g/g) was calculated using Equation (7):
Water swelling capacity
Water swelling capacity (WSC) was assessed using the method described by Ma & Mu [2016]. Briefly, 0.2 g of dried SDF preparation (W) was mixed with 10 mL of distilled water in a graduated 15-mL tube and equilibrated at room temperature (25°C) for 18 h. The volume of the sample before (V1) and after (V2) hydration was recorded. The WSC (mL/g) was calculated using Equation (8):
Emulsifying capacity and emulsion stability
The emulsifying capacity (EC) was evaluated following the method of Nandi & Ghosh [2015] with slight modifications. Specifically, 0.5 g of dried SDF preparations was dispersed in 50 mL of distilled water and homogenized at 10,000 rpm for 1 min. Subsequently, 50 mL of soybean oil was added, and the mixture was homogenized again at the same speed and duration. The resulting emulsion was centrifuged at 2,800×g for 5 min. After centrifugation, the total volume of the system (Wv) and the volume of the emulsion layer (ELvi) were recorded. The EC (%) was then calculated using Equation (9):
To determine emulsion stability (ES), the freshly prepared emulsion (from the EC determination) was heated at 80°C for 30 min, then allowed to cool to room temperature (25°C) and centrifuged at 2,800×g for 5 min. The volume of the remaining emulsified layer after heating (ELvf) was recorded. ES was calculated as a percentage of the initial emulsion volume (ELνi) using Equation (10):
Analysis of structural characteristics
The SDF preparations were analyzed for their structural characteristics using Fourier-transform infrared spectroscopy (FTIR), X-ray diffraction (XRD), and scanning electron microscopy (SEM). FTIR analysis was conducted on a Frontier NIR/MIR spectrometer (Perkin Elmer, Waltham, MA, USA) within the wavelength range of 400–4,000 cm⁻1 to identify the functional group vibrations. For sample preparation, dried powder was mixed with potassium bromide (KBr) in a 1:40 ratio by weight and compressed into 1-mm thick pellets using a hydraulic press. Each sample was scanned 20 times to ensure reliable spectral resolution.
XRD analysis was carried out using an Empyrean X-ray diffractometer (PANalytical, Almelo, Netherlands) equipped with Cu K-α radiation (λ=1.5406 Å), operating over at 40 kV and 40 mA. Diffraction patterns were recorded over a 2θ range of 5–80° at a step size of 0.02° and a counting time of 0.5 s per step. The primary aim of this analysis was to evaluate the crystalline versus amorphous nature of the extracted fiber samples. The crystallinity index (CI) was calculated based on the integrated intensities obtained from the diffractograms. Specifically, the CI (%) was determined using Equation (11) [Salem et al., 2023]:
where: Ac is the integrated area under the crystalline peaks, Aa is the integrated area under the amorphous halo or background, and (Ac+Aa) represents the total area under the XRD curve.
Peak fitting and area integration were performed using HighScore Plus software (PANalytical), allowing separation of the crystalline and amorphous contributions by deconvoluting overlapping peaks. This approach enables an accurate estimation of the relative crystallinity by quantifying the ratio of the crystalline signal to the total diffracted intensity.
For microstructural examination, SEM images were obtained using a JSM IT 200 field-emission scanning electron microscope (Jeol, Tokyo, Japan) operated at an accelerating voltage of 10 kV. Prior to imaging, the samples were affixed to double-sided conductive adhesive tape and sputter-coated with platinum to enhance conductivity and image clarity. SEM images were captured at magnifications of 100×; 1,000×; and 7,000×.
Statistical analysis
All data collected were based on the results of at least three replicates for each experiment and are expressed as mean ± standard deviation. Statistical analyses were performed using Statgraphics Centurion 16.1 software (StatPoint Technologies, Inc., Warrenton, VA, USA). Analysis of variance (ANOVA), followed by the least significant difference (LSD) test, was used to determine significant differences (p<0.05) between SDF preparations obtained under different conditions.
RESULTS AND DISCUSSION
Extraction efficiency and fiber composition
The extraction efficiency of orange by-products and composition of soluble dietary fiber preparations obtained using various extraction methods, including extraction under acid and alkaline conditions (resulting in A-SDF and B-SDF, respectively, and enzyme-assisted extraction with cellulase and xylanase (resulting in EC-SDF and EX-SDF, respectively), is shown in Table 1. The EE varied significantly (p<0.05) depending on the extraction method. The highest EE was achieved with chemical extraction methods: 31.37% with acid extraction and 24.18% with alkaline extraction. This was followed by the cellulose-assisted method (16.88%), while the lowest extraction efficiency was obtained using the xylanase-assisted method (13.88%). The significantly higher EE of A-SDF method suggests that citric acid extraction was more effective at solubilizing orange by-product matter than enzymatic treatments. Citric acid may facilitate the breakdown of the plant matrix and improve fiber solubilization, as observed in previous studies [Maran et al., 2017]. Enzymatic methods resulted in the lowest EE, possibly due to the selective hydrolysis of specific fiber components, leading to reduced overall recovery [Canela-Xandri et al., 2018]. For instance, cellulase acts on β-1,4 glycosidic bonds, whereas xylanase breaks the bonds between hemicellulose and cellulose, leading to a lower extraction efficiency but a higher SDF content due to the breakdown of cellulose and hemicellulose into soluble fibers [Song et al., 2021; Zhao et al., 2025; Zheng & Li, 2018].
Table 1
Extraction efficiency of orange by-products and composition of soluble dietary fiber (SDF) preparations obtained by different methods.
[i] Data are presented as the mean ± standard deviation. Different letters in the same column indicate statistically significant differences between preparations (p<0.05). A-SDF, SDF preparation obtained under acid conditions; B-SDF, SDF preparation obtained under alkaline conditions; EC-SDF, SDF preparation obtained by cellulose-assisted extraction; EX-SDF, SDF preparation obtained by xylanase-assisted extraction; dm, dry matter; IDF, insoluble dietary fiber; TDF, total dietary fiber.
For total dietary fiber (TDF), significant differences (p<0.05) were observed among the preparations obtained by different extraction methods (Table 1). EC-SDF exhibited the highest TDF content (66.81 g/100 g dm), significantly greater than all the other samples. EX-SDF showed a higher TDF content (66.58 g/100 g dm) compared to A-SDF and B-SDF, whose TDF contents were 66.09 and 66.17 g/100 g dm, respectively. These findings suggest that enzymatic treatments, particularly those with cellulase and xylanase, enhanced the total fiber recovery compared to acid and alkaline extractions. Moreover, there were substantial differences in the content of IDF and SDF fractions. B-SDF and EXSDF had the highest IDF content (25.13 and 22.10 g/100 g dm, respectively), whereas EC-SDF had the highest SDF content (50.11 g/100 g dm), followed by A-SDF (48.24 g/100 g dm). This indicated that cellulase treatment effectively converted IDF into SDF, which is consistent with the findings of Zheng & Li [2018]. In terms of specific fiber components, EX-SDF exhibited the highest cellulose content (11.73 g/100 g dm), indicating that xylanase-assisted extraction preserved the cellulose structure while degrading hemicellulose. B-SDF had the highest hemicellulose content (7.58 g/100 g dm), suggesting that alkaline treatment effectively preserved hemicellulose. Notably, EC-SDF had the highest pectin content (61.22 g/100 g dm), followed by A-SDF and B-SDF (58.31–58.63 g/100 g dm). The EX-SDF pectin content was the lowest (56.24 g/100 g dm). The higher pectin content in EC-SDF supported the role of cellulase in selectively breaking down cellulose while preserving pectin. In turn, a high pectin content contributes to enhanced functional properties, such as water-holding capacity and water-swelling capacity relevant to food applications [Huang et al., 2021]. In conclusion, extraction under acid conditions provided the highest yield and maintained a good balance between SDF and IDF of A-SDF. In turn, alkaline extraction was proved suitable for obtaining preparations for applications that require a higher IDF content. Among all methods, cellulase treatment increased the content of SDF and pectin the most, making EC-SDF ideal for functional foods, whereas xylanase-assisted method preserved cellulose but resulted in lower EE. These findings highlight the influence of extraction method on fiber yield and composition, consistent with trends observed in other studies using enzymatic or chemical extraction techniques [Dong et al., 2020; Huang et al., 2021; Zhang et al., 2017; Zhao et al., 2025].
Functional properties
The physicochemical properties of SDF preparation obtained from orange by-products using different extraction methods, including water solubility, water holding capacity, oil holding capacity, water swelling capacity, emulsifying capacity, and emulsifying stability, are shown in Table 2. A-SDF exhibited the highest WHC (19.17 g/g), which was significantly different (p<0.05) from the other samples. The WHC of B-SDF, EC-SDF, and EX-SDF ranged from 4.55 to 5.51 and the values did not differ significantly (p≥0.05) from each other. The WHC of A-SDF was higher than that reported for orange peel soluble dietary fiber extracted using steam explosion and sulfuric-acid soaking method – 5.52 g/g [Wang et al., 2015]. In turn, Huang et al. [2021] found higher WHC, compared to the value in our study for B-SDF, for untreated citrus peel (8.32 g/g) and treated under different alkaline conditions (18.26–21.32 g/g) compared to the value in our study for B-SDF. Generally, WHC is enhanced by chemical and enzymatic treatments that disrupt hydrogen bonds in cellulose and hemicellulose, de-esterify pectin, and expose hydrophilic groups, thereby increasing the fiber’s water absorption capacity [Liu et al., 2021; Zhang et al., 2020].
Table 2
Physicochemical properties of soluble dietary fiber (SDF) preparations obtained from orange by-products using different methods.
[i] Data are presented as mean ± standard deviation. Different letters within the same column indicate statistically significant differences between preparations (p<0.05). A-SDF, SDF preparation obtained under acid conditions; B-SDF, SDF preparation obtained under alkaline conditions; EC-SDF, SDF preparation obtained by cellulose-assisted extraction; EX-SDF, SDF preparation obtained xylanase-assisted extraction; WS, water solubility; WHC, water holding capacity; OHC, oil holding capacity; WSC, water swelling capacity; EC, emulsifying capacity; ES, emulsion stability.
The fiber hydrolyzed by xylanase exhibited a higher swelling capacity (8.67 mL/g), while the alkaline treatment resulted in the lowest WSC (4.92 mL/g), with a significant (p<0.05) difference observed between the samples (Table 2). This result was consistent with previous studies, which suggested that WSC depends on the soluble fiber content [Navarro-González et al., 2011], particularly due to the exposure of small-sized pectin with a large surface area, allowing for the formation of hydrogen bonds that promote water absorption and swelling [Ma & Mu, 2016; Navarro-González et al., 2011]. The lower WSC in the alkaline-treated samples could also be related to the disruption of the matrix structure and the breakdown of polysaccharide linkages under alkaline conditions [Jiang et al., 2020].
The OHC of dietary fiber is important for improving the texture and mouthfeel of food products and may also assist in retaining lipophilic nutrients during formulation [Marczak & Mendes, 2024]. Additionally, dietary fiber with high OHC has been associated with potential cholesterol-lowering effects due to its ability to bind bile acids and fats [Elleuch et al., 2011]. In this study, OHC ranged from 1.34 to 2.49 g/g (Table 2). These values were comparable to those obtained in previous studies on citrus peel-derived dietary fiber, which reported OHC ranging from 2.08 to 5.0 g/g, depending on the extraction method and fiber structure [Huang et al., 2021; Zhang et al., 2020; Zhao et al., 2025]. Among the tested samples, the OHC of A-SDF, EC-SDF, and EXSDF ranged from 2.38 to 2.49 g/g and did not differ significantly (p≥0.05), suggesting that both acid and enzyme-assisted extractions were similarly effective in preserving oil-binding functionality. In contrast, B-SDF exhibited a significantly (p<0.05) lower OHC (1.34 g/g), indicating that alkaline treatment may reduce the fiber’s ability to retain oil. This reduction could be attributed to structural degradation or loss of hydrophobic binding sites during alkaline hydrolysis. The relatively higher OHC observed in the acid- and enzymatic-treated fibers may be due to a more porous surface morphology and greater exposure of lipophilic groups, which facilitate oil entrapment within the fiber matrix [Dong et al., 2020; Zhang et al., 2020].
The WS of SDF preparations obtained from orange by-products ranged from 50.05 to 76.20 (g/g). While water solubility is often influenced by the soluble fiber content, no strong correlation was observed in this study. Notably, EC-SDF exhibited the highest SDF content (50.01 g/100 g dm) but showed relatively low WS (54.17 g/g). This discrepancy may be attributed to the structural characteristics of the extracted polysaccharides. Enzymatic degradation likely produced smaller molecular weight fragments, including low-methoxyl pectin, which may have altered solubility behavior and increased solution viscosity, thereby affecting dispersibility and solubility kinetics [Kermani et al., 2014; Song et al., 2021].
Emulsifying capacity refers to the ability of a substance to facilitate the dispersion of two immiscible liquid phases, while emulsion stability indicates to maintain the stability of the formed emulsion over time [Dong et al., 2020]. The A-SDF, B-SDF, EC-SDF, and EX-SDF preparations exhibited EC of 91.00, 59.17, 42.94, and 3.00%, and corresponding ES of 79.40, 53.52, 9.32, and 75.77%, respectively (Table 2). Interestingly, EX-SDF demonstrated a relatively high ES despite its extremely low EC. The intense degradation induced by xylanase likely enhanced its water swelling capacity due to increased porosity, allowing more water to be absorbed into the fiber matrix. However, the re-agglomeration of fragmented structures into denser, more ordered forms may have reduced the available surface area, contributing to a lower water holding capacity and hindering effective contact between the oil and water phases-thereby reducing EC. SDF preparations obtained through enzyme-assisted extraction generally exhibited lower EC compared to those extracted chemically (Table 2). This difference could be explained by the extensive disruption of cellulose and hemicellulose during enzymatic treatment, which may impair both hydration ability and oil-binding performance, ultimately diminishing emulsifying potential [Zheng & Li, 2018]. The emulsifying behavior of SDF is influenced by multiple factors, including WHC, OHC, fiber structure, and surface characteristics [Jia et al., 2019; Zhang et al., 2017]. Among these factors, the ability of dietary fibers to retain water (WHC) and bind oil (OHC) is particularly important, as it enhances the fiber’s ability to stabilize oil–water interfaces in emulsions.
Structural characteristics
Characteristic functional groups of the soluble dietary fiber preparations
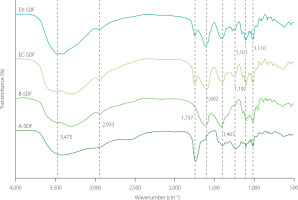
The FTIR spectra revealed that all the SDF preparations exhibited similar characteristic spectra, as shown in Figure 1. The SDF samples exhibited strong absorption in the 3,300–3,500 cm⁻1 region, with a distinct absorption peak at 3,473 cm⁻1, corresponding to the O–H group’s hydrogen bond vibration in polysaccharides [Dong et al., 2020]. Compared to A-SDF, shifts in the absorption band and a reduction in peak intensity observed in B-SDF and EC-SDF suggest the disruption of hydrogen bonds in the cellulose and hemicellulose chains [Song et al., 2021]. Additionally, a slight increase in intensity may be attributed to the formation of hydrogen bonds between the hemicellulose and pectin chains [Zhang et al., 2020]. All citrus fibers exhibited C–H stretching vibrations from the –CH2 and –CH3 groups of polysaccharides at approximately 2,933 cm⁻1 [Song et al., 2021; Yan et al., 2015]. Furthermore, the peak at 2,933 cm⁻1 is associated with the intermolecular hydrogen bonding of the –OH group in cellulose [Jia et al., 2019]. The decreased absorption intensity at 2,933 cm⁻1 in the modified citrus fibers indicates a reduction in the polysaccharide content and disruption of hydrogen bonds between –OH groups in the cellulose structure, which could enhance the functional properties of citrus fibers. A distinct absorption peak at approximately 1,737 cm⁻1 was assigned to the C=O stretching of hemicellulose, pectin, or lignin [Zhang et al., 2020], but this peak disappeared after alkaline hydrolysis [Jiang et al., 2005]. The characteristic wavenumber position for the C=O vibration of the acetyl and uronic ester groups in hemicellulose, observed at approximately 1,737 cm⁻1, was present in all samples but disappeared after alkaline hydrolysis, as the alkaline medium solubilizes both hemicellulose and lignin [Jiang et al., 2005]. Peaks at 1,602 cm⁻1 and 1,401 cm⁻1, corresponding to the aromatic benzene ring vibrations associated with the bending or stretching of aromatic hydrocarbons in lignin [Ma & Mu, 2016; Song et al., 2021], were detected in all samples. The weaker signal observed for A-SDF may indicate that lignin was partially precipitated in the acidic medium. Additionally, the wavenumber ranges between 1,000 and 1,200 cm⁻1 are characteristic of the C–O and C–C stretching vibrations in the glycan core [Dong et al., 2020].
Amorphous and crystal structural characteristics of soluble dietary fiber preparations using X-ray diffraction
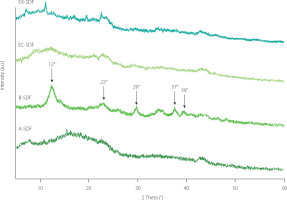
The XRD patterns of the SDF preparations are shown in Figure 2, where the fibers processed by all four extraction methods exhibited similar main peaks at 12° and 22°, indicating that all samples contained type I cellulose [Song et al., 2021; Zhang et al., 2020]. The narrow peak positions and widths of the B-SDF and EX-SDF samples suggest that the ordered crystalline regions were not significantly disrupted by enzymatic hydrolysis with xylanase or by alkaline treatment [Jiang et al., 2020]. In contrast, A-SDF and EC-SDF exhibited broader peak widths, indicating a more amorphous structure in these fibers. The crystallinity index (CI) of A-SDF, B-SDF, EC-SDF, and EX-SDF was 34.64, 41.83, 30.13, and 37.43%, respectively. The higher CI of the citrus fiber after alkaline treatment is likely due to the degradation of some amorphous components, such as pectin or lignin [Zhang et al., 2020]. The lower CI determined for EC-SDF compared to the other preparations suggests that cellulase hydrolysis may have disrupted the ordered cellulose structure. This enzymatic action is known to break hydrogen bonds and degrade crystalline regions, converting them into more amorphous forms [Hassan et al., 2018; Jiang et al., 2020]. The decrease in (hemi)cellulose content, as was shown in Table 1, further demonstrates the solubilization of these components during cellulase treatment.
Microstructural characteristics of soluble dietary fiber preparations
The morphological structures of SDF preparations obtained by different methods revealed notable differences under SEM (Figure 3). SDF produced through acid extraction (A-SDF) displayed smaller particle with rougher and more porous surfaces. These characteristics contributed to its superior water absorption, resulting in significantly higher water-holding capacity and water swelling capacity compared to the EC-SDF, EX-SDF and B-SDF fibers (Table 2). The irregular shapes provide a greater surface area for interaction with water molecules. Moreover, the coarse surface may enhance emulsifying capacity (EC) by offering additional sites for interaction with oil droplets. Citric acid extraction primarily solubilizes pectin, moderately disrupting the matrix and preserving the fiber network-likely due to limited hydrolysis of polysaccharides [Maran et al., 2017].
Figure 3
Scanning electron microscopy images of soluble dietary fiber preparations obtained from orange by-products by different methods including (A) acid extraction (A-SDF), (B) alkaline extraction (B-SDF), (C) cellulose-assisted extraction (EC-SDF), and (D) xylanase-assisted extraction (EX-SDF).
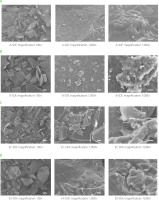
In contrast, the SDF preparation obtained with sodium hydroxide showed a highly fragmented and irregular structure (Figure 3B). At higher magnifications (1,000× and 7,000×), the surfaces appeared smoother and less porous, suggesting a reduction in structural integrity. Alkaline treatment resulted in intense fiber degradation, as evidenced by B-SDF significantly lower water swelling capacity (4.92 mL/g) and soluble dietary fiber content (41.03 g/100 g dm) compared to the other preparations (Tables 1 and 2). This reduction in functional properties is likely due to compromised fiber porosity and solubility. Furthermore, B-SDF exhibited the lowest oil-holding capacity (1.34 g/g), which may be attributed to its smoother and more compact surface structure. Similar effects of alkaline degradation on fiber microstructure and functionality have also been reported by Ma & Mu [2016]. The SDF preparation obtained through cellulase hydrolysis exhibited a distinctly broken fibrous network, particularly evident at the 2 µm scale (Figure 3C). The enzymatic breakdown of cellulose resulted in enhanced surface roughness and increase porosity, which contributed to better water absorption and water swelling capacity. Among all preparations, EX-SDF showed the most pronounced fiber disintegration. SEM images (Figure 3D) revealed a highly porous and irregular network formed by extensive hydrolysis, indicating the strong action of xylanase on hemicellulose. While the high porosity suggested potential for improved hydration and swelling, EX-SDF actually recorded the lowest WHC among all samples (Table 2). This paradox may be explained by excessive fiber breakdown and surface aggregation of small molecules, which reduce the effective surface area for water retention. Furthermore, the fragmented and disordered structure may have hindered oil binding and emulsification, due to the lack of continuous surface needed for stable interactions [Jia et al., 2019]. Interestingly, the fiber surface in EX-SDF also appeared denser in some regions with rounded voids, possibly resulting from the re-aggregation of degraded fragments into a more compact structure. This reorganization may have reduced water accessibility, thereby lowering the WHC of the enzyme-treated samples compared to the acid-treated ones (Table 2). Similarly, Song et al. [2021] reported that enzymatic modification can disrupt fiber structure and reduce WHC due to the breakdown of organized polysaccharide networks.
Thus, the morphological differences among SDF samples may be related to differences in their functional properties, including hydration, solubility, oil adsorption, and emulsifying behaviors. These findings align with previous reports that associate fiber porosity with water retention properties [Dong et al., 2020]. Based on the observed microstructural characteristics, acid extraction appears to be the most effective method for enhancing the hydration capacity of soluble dietary fiber.
CONCLUSIONS
The findings of this study demonstrate that the structural and functional properties of SDF extracted from C. sinensis peels vary significantly depending on the extraction method used, including acid, alkaline, and enzymatic treatments. Each method imparted distinct characteristics to the SDF preparations, highlighting their potential for tailored applications in food formulations. The choice of extraction method had a pronounced influence on fiber yield, structural morphology, and physicochemical properties. Among the approaches evaluated, the use of citric acid provided the highest extraction efficiency and superior water-holding and emulsifying properties, attributed to the increased porosity and solubility of the resulting SDF preparations. Alkaline extraction resulted in fiber with the highest crystallinity index, indicating a more ordered structure. Enzymatic treatments led to preparations with high oil holding capacity, but in general, these preparations showed variable properties depending on the enzyme used. The properties (WS, OHC, WSC, ES, CI) of the fiber produced by xylanase-assisted extraction were similar to those of fiber obtained under acidic conditions. These results underscore the specific advantages of each method depending on targeted application. The highest water-holding capacity and emulsion stability of citric acid-extracted fiber indicates its suitability for applications focused on moisture retention and emulsion stabilization. Future studies should explore the industrial-scale applications and focus on scaling up these extraction techniques and evaluating the nutritional functionalities, including especially the prebiotic potential of the resulting fiber. Ultimately, the optimized utilization of C. sinensis peels as a dietary fiber source offers a promising strategy for sustainable food production and agro-industrial waste valorization.