INTRODUCTION
The beetroot (Beta vulgaris L.) is a plant of the Chenopodiaceae family that is native to Asia and Europe [Chhikara et al., 2019]. Its roots are a popular vegetable rich in carotenoids, phenolics, betalains, ascorbic acid, and B-group vitamins (thiamine, riboflavin, niacin, pantothenic acid, pyridoxine, folic acid), and they also provide fiber, low-energy sugars, and inorganic nitrate [Chhikara et al., 2019; Clifford et al., 2015]. Beetroots are also a good source of minerals, including manganese, magnesium, potassium, sodium, phosphorus, iron, zinc, and copper [Mirmiran et al., 2020]. Due to the abundance and diversity of health-promoting compounds, beetroots have antioxidant, anti-inflammatory, anticancer, and antidiabetic properties, and they may also contribute to reducing the risk of development of cardiovascular diseases and hypertension [Chhikara et al., 2019; Mirmiran et al., 2020]. These healthy vegetables have a unique taste and can be consumed fresh, cooked, and fermented [Clifford et al., 2015; Sawicki & Wiczkowski, 2018].
During lactic acid fermentation, lactic acid bacteria convert plant carbohydrates to lactic acid, which lowers the pH of fermented products. In turn, the lower pH inhibits the growth of some spoilage flora and pathogenic bacteria [Montet et al., 2014]. Fermentation modifies the organoleptic characteristics of raw materials, including flavor and taste, and enhances the safety, nutritional value, sensory attributes, and the shelf-life of raw foods [Czyżowska et al., 2020; Montet et al., 2014]. Moreover, lacto-fermentation can activate, transform, and increase the availability of bioactive compounds by softening and promoting the degradation of the food matrix, which enhances the health-promoting effects of these compounds [Daliri et al., 2023; Sawicki & Wiczkowski, 2018; Zhao et al., 2021]. Currently, there is a growing interest in products prepared from lactofermented vegetables because consumers have a preference for natural food preservation methods that increase the health benefits provided by these products. Studies have shown that fermented products can prevent and support the treatment of metabolic disorders and cardiovascular diseases, improve cognitive functions, and boost immunity [Sivamaruthi et al., 2018].
Unusually for fermented products, a fermented beetroot solution is more popular than fermented beetroot tissue (especially in Central and Eastern Europe) because it has anti-carcinogenic and anti-mutagenic potential, promotes the growth of beneficial gut bacteria, and reduces the risk of development of diseases related to food intolerance [Mirmiran et al., 2020]. In many cases, lacto-fermented beetroot juice is considered the main product, while fermented beetroot tissue is regarded as a by-product of the fermentation process. However, fermented beetroot tissue can also be valued for its high nutritional value and health benefits. Unfortunately, these vegetables are highly susceptible to mold spoilage due to their high water content. For this reason, after lactic acid fermentation, beetroots should be subjected to additional preservation treatments, such as freezing and/or dehydration (osmotic and/or convective). These treatments can be applied to obtain fermented beetroot chips, a novel food product that may be attractive for consumers due to its health benefits and unique taste.
Freezing is an effective food preservation method, but it can also be applied as a pre-treatment technique to improve drying efficiency. During the process, plant cell walls are damaged by ice crystals growing inside cells. By shortening drying time, freezing also minimizes the need for thermal processing, thus protecting thermolabile nutrients and increasing the nutritional value of the product [Zielinska et al., 2019; Zielinska & Zielinska, 2019]. In beetroots subjected to convective drying (temperature of 40°C; airflow rate of 1 m/s), the freezing pre-treatment (at −20°C) significantly shortened drying time (by 46%). However, it had no significant effect on the content of betaxanthins and betacyanins in dried beetroots but led to a significant reduction in the content of phenolic compounds and antioxidant activity compared to beetroots dried without pre-freezing [Vallespir et al., 2018].
Other food drying techniques include osmotic dehydration and pulsed vacuum osmotic dehydration (PVOD) [James et al., 2014]. In raw beetroots, osmotic pre-treatment (using NaCl solution) shortened drying time, decreased water activity, promoted the retention of betanin, enhanced color preservation, and increased water resorption [Kowalski & Łechtańska, 2015]. The use of a sucrose solution in osmotic dehydration and contact drying in the refractance window technique generated similar benefits, where drying time was shortened with a rise in the concentration of the solution and the temperature of osmotic dehydration [Calderón-Chiu et al., 2020]. Studies have shown that the PVOD method reduces the initial amount of water in the processed material, thus shortening the subsequent drying time [Corrêa et al., 2021; Pan et al., 2003]. Despite the fact that the impact of PVOD on fruit drying has been extensively studied, the influence of PVOD on the drying kinetics and the physicochemical properties of fermented beetroots has not been analyzed to date. The existing research focused mainly on the impact of PVOD on the physicochemical properties of beet tissue [Staniszewska et al., 2024]. However, the extent to which this processing technique affects drying kinetics and the physicochemical properties of dried fermented beetroots remain unknown.
Ultrasonic treatments can be applied both before (as a pretreatment) and during the drying process (as ultrasound-assisted drying) to enhance and shorten convective drying of fruits and vegetables. Both techniques can accelerate the drying process [Fijałkowska et al., 2015; Szadzińska et al., 2020]. However, their effectiveness can be affected by some process variables, including air velocity and air temperature [Huang et al., 2020]. Moreover, the effect of ultrasonic treatment on the nutritional value and technological and functional properties of foods should be evaluated separately for each product [Soria & Villamiel, 2010]. To the best of the authors’ knowledge, there are currently no published studies on fermented beetroots that were additionally frozen or osmotically dehydrated and dried using airborne high-power ultrasound (HPU).
Therefore, the aim of this study was to evaluate the effects of ultrasound-assisted drying on the drying kinetics and selected nutritional and functional properties of raw, fermented, frozen, and osmotically dehydrated fermented red beetroots relative to beetroots subjected only to convective drying.
MATERIALS AND METHODS
Material
Fresh beetroots (Beta vulgaris L.) were obtained from a local grocery wholesaler (Olsztyn, Poland). Their initial moisture content was 5.93±0.27 g H2O/g dry matter (DM) [AOAC, 2002]. The vegetables were ripe, free of apparent diseases, and similar in terms of freshness and size. Before the experiments, beetroots were cleaned under tap water and cut into slices with a thickness of 4±1 mm. Raw beetroot slices (R) were divided into groups and subjected to various treatments described below. The list of treatments and the codes of products obtained as a result of these treatments are presented in Table 1.
Table 1
Codes of beetroot products.
Fermentation
Beetroot slices (5 kg) and spices, i.e., peppercorns (2.5 g), allspice (5.0 g), bay leaves (4.5 g), and garlic cloves (125 g), were placed in a glass jar, and the jar was filled with a 1.8% aqueous sodium chloride (NaCl) solution. The sample-to-solution ratio was 1:1.2 (w/v). The jar was closed and left to ferment in darkness. Beetroots were fermented at 20°C for 7 days. A similar fermentation method was used by Staniszewska et al. [2024].
Osmotic dehydration
Fermented beetroot slices (F) were subjected to osmotic dehydration (O) in a vacuum dryer (DZ ZBC II, Chemland, Stargard Szczecinski, Poland) at a temperature of 20°C. The osmotic solution was an aqueous solution composed of 60% sucrose and 5% sodium chloride. Initially, the material immersed in the osmotic solution was subjected to vacuum (−0.09 MPa) for 10 min, and then dehydrated at an atmospheric pressure for up to 180 min. The material-to-solution ratio during osmotic dehydration was 1:4 (w/v). Osmotic dehydration parameters approximated the optimal values described in the literature [Staniszewska et al., 2024].
Freezing and thawing
Fermented beetroot slices subjected to the freezing/thawing treatment (FF) were frozen at −18°C using a GT 4932 Comfort freezer (LIEBHERR, Bischofshofen, Austria). Before drying, they were thawed at room temperature for approximately 24 h.
Drying
Beetroot slices were subjected to hot air convective drying (C), both with and without high power ultrasound (HPU), in a laboratory hybrid dryer (SLH-2, PROMIS TECH, Wroclaw, Poland) at a temperature of 80°C with an air flow rate of around 1 m/s. A single layer of beetroot slices (approx. 200 g) was spread on a mesh tray mounted on a digital scale (ACZ6200, Axis sp. z o.o., Gdańsk, Poland). Airborne ultrasound (200 W) was applied during ultrasound-assisted drying experiments. During convective drying with ultrasound assistance (CUS), HPU was used continuously in the following cycles: 10 min without ultrasound and 5 min with ultrasound assistance. The mass of dried samples (±0.01 g) was monitored and registered at 5 min intervals. The convective drying treatment was continued until no further decrease in beetroot weight was observed in two subsequent measurements which meant that equilibrium moisture content was achieved. The drying kinetics curve was plotted based on the results of a single experiment for a given treatment. The moisture content of beetroot samples before and after drying was determined in a vacuum drying oven (DZ ZBC II, Chemland, Stargard Szczeciński, Poland) at 70°C and 13.3 kPa for 24 h [AOAC, 2002] and its value was calculated by dividing the water loss by the DM content. The water loss was the difference between the weight before and after drying. The drying rate was calculated as the difference between the initial and final moisture content divided by drying time and expressed as g H2O/(g DM×min). The energy consumption was calculated by dividing the total energy consumption registered by the drier during the whole process by the water loss.
Microstructure and color analysis
The surfaces of dried beetroot slices were examined under a digital microscope with imaging software (VHX-7000, Keynes, Osaka, Japan) at 500× and 1,000× magnification. Images were acquired at 4k resolution under full illumination.
The color of raw and dried beetroot slices was measured with a spectrophotometer (MiniScan XE Plus, Hunter Associates Laboratory Inc., Reston, USA) under standard illuminant D65, 10° observer and 8° diaphragm, and was expressed in CIE L*a*b* space, where L*, a*, and b* represent lightness, (+)redness/(−)greenness, and (+)yellowness/(−)blueness, respectively. Total color differences (ΔE) between the samples were calculated according to Equation (1) presented below, taken from the literature [Zielinska & Markowski, 2012]. Three types of total color differences were calculated: ΔE1 – total color difference relative to raw beetroot slices (R), ΔE2 – total color difference relative to raw beetroot slices dried by hot air (RC), and ΔE3 – total color difference between the same samples dried with and without ultrasound. The color of beetroots was measured directly on the surface. The results were averaged over 20 measurements.
Total phenolic content, antioxidant capacity, and sugar content analysis
Pulverized dried beetroot slices (approx. 0.05 g) were extracted by sonication with 1 mL of acidified 80% (v/v) methanol (with 0.1% HCl, v/v) using a VC 750 ultrasonic processor (Sonics & Materials, Newtown, CT, USA) for 30 s. Each sample was initially vortexed for 30 s, followed by alternating cycles of sonication and vortexing to enhance the extraction efficiency. After each cycle, the mixture was centrifuged at 13,200×g for 10 min using an Eppendorf centrifuge 5424 (Eppendorf, Wesseling-Berzdorf, Germany). This process was repeated a total of five times, with 1 mL of fresh solvent added at each step to ensure thorough extraction of the analytes. The resulting supernatants were pooled and stored at –20°C until analysis, which was carried out within 24 h [Zielinska & Zielinska, 2019]. The extraction procedure was carried out in triplicate.
The total phenolic content (TPC) and the ferric reducing antioxidant power (FRAP) were determined by Singleton et al. [1999] and Benzie & Strain [1996] methods, respectively. In the first assay, the appropriately diluted extract (90 μL), the Folin–Ciocalteu reagent diluted with water (1:1, v/v), and the saturated sodium carbonate solution were mixed in a ratio of 1:1:2 (v/v/v) using a thermomixer (Comfort, Eppendorf). Then, the volume of the mixture was adjusted to 2 mL with water. The mixture was allowed to stand for 25 min at ambient temperature and then the absorbance was measured at 725 nm using a UV-1800 spectrophotometer (Shimadzu Corporation, Kyoto, Japan). Gallic acid was used as the standard, and the results were expressed as mg of gallic acid equivalents (GAE) per g of DM. For the FRAP assay, 100 µL of the diluted extract were mixed with 3 mL of a freshly prepared FRAP reagent (a mixture of 300 mM acetate buffer pH 3.6, 10 mM 2,4,6-tris(2-pyridyl)-s-triazine in 40 mM HCl, and 20 mM FeCl3×6H2O in a 10:1:1, v/v/v, ratio). After mixture incubation at 37°C for 10 min, the absorbance was measured at 593 nm. FRAP was expressed in mg Trolox equivalents (TE) per g of DM. The assays were performed in triplicate.
Sugar content (SC) was determined by the spectrophotometric method [Nielsen, 2017]. The sample was prepared by heating 10 g of pulverized dried beetroot slices in 300 mL of distilled water for 10 min with stirring in a SW22 Julabo shaking water bath (JULABO GmbH, Seelbach, Germany). Then, 5 mL of 5% phenol and 1.0 mL of distilled water were added to the samples, which were then stirred for 1 min. In the next step, 5.0 mL of concentrated sulfuric acid was added, and the samples were shaken for 3 min. The resulting solution was settled for 30 min, cooled with water for 20 min, and absorbance was measured at 490 nm using an LKB Ultraspec II spectrometer (Pharmacia LKB Biotechnology, Piscataway, NJ, USA). Standard solutions containing a specific amount of d-glucose (Chempur, Piekary Śląskie, Poland) were prepared for the determination of sugar content. The measurements were performed five times for each beetroot product.
Fourier-transform infrared spectroscopy analysis
For each beetroot product, Fourier-transform infrared spectroscopy (FTIR) spectra were obtained in triplicate using a Perkin Elmer Spectrum Two device (Perkin Elmer, Waltham, MA, USA) equipped with a diamond attenuated total reflectance (ATR) gear. The spectra were registered at a resolution of 1 cm−1 within the wavenumber range of 4,000–450 cm−1 at room temperature.
Statistical analysis
Differences between beetroot products were determined by one-way analysis of variance (ANOVA) with post-hoc Duncan’s test (for ΔE), the non-parametric Kruskal–Wallis test (for FRAP and TPC), and the Mann–Whitney U test (for L*, a*, b*, water content, and sugar content), at a confidence level of 95% (statistical significance at p<0.05). Calculations were performed using Statistica 13.0 software (TIBCO Software Inc., Santa Clara, CA, USA).
RESULTS AND DISCUSSION
Drying kinetics of beetroot slices
The basic parameters of beetroot slice drying, including drying time, final water content, water loss (evaporated water), drying rate, and energy consumption, are shown in Table 2. The use of high-power ultrasound (HPU) in a pulse mode during the convective drying exerted the greatest impact on raw beetroot slices by shortening drying time from 183 min (RC) to 140 min (RCUS), i.e., by 23.5%, and reducing specific energy consumption from 102.52 kJ/g H2O to 83.70 kJ/g H2O, i.e., by 18.4%. The drying rate increased from 0.0318 g H2O/(g DM×min) (RC) to 0.0409 g H2O/(g DM×min) (RCUS), which points to a 28.6% improvement in process efficiency. The drying speed was higher in the first stage of the process, as indicated in Figure 1A. The increase in drying rate, the decrease in drying time, and the increase in energy efficiency can be attributed to the cavitation effect which increased the porosity of the material and facilitated the migration of water from the interior to the surface [Mason et al., 1996]. Szadzińska et al. [2020] reported a similar significant decrease in the drying time of beetroot cubes (15 mm side length) subjected to HPU-assisted drying (200 W) at a temperature of 60°C and airflow velocity of 2 m/s relative to convective drying. These authors suggested that ultrasound improved heat and mass transfer through cavitation, which led to faster evaporation of water and shortened drying time. Ultrasound also disrupted plant cell walls, which promoted water flow from the interior to the surface of the material. Szadzińska et al. [2020] found that energy consumption was also lower during HPU-assisted drying than convective drying of raw beetroots, although its value was significantly higher than in our case, which could be attributed to a much longer drying time resulting from lower air temperature and thicker samples.
Table 2
Basic drying parameters of a 200-g batch of beetroot slices exposed to different treatments.
[i] Results are shown as mean ± standard error. Identical letters in the same column denote the absence of significant differences (p≥0.05). R, raw; F, fermented; O, osmotically dehydrated; FF, fermented and frozen/thawed; C, convective dried; CUS, convective dried with ultrasound assistance; DM, dry matter.
Figure 1
Drying kinetics of beetroot slices with (CUS) and without (C) ultrasound assistance: (A) drying of raw beetroot slices (R); (B) drying of fermented beetroot slices (F); (C) drying of fermented and frozen/thawed beetroot slices (FF); (D) drying of fermented and osmotically dehydrated beetroot slices (FO); (E) drying of fermented, frozen/thawed and osmotically dehydrated beetroot slices (FFO).

The drying time of fermented and osmotically dehydrated beetroot slices was also significantly shortened by the HPU treatment, from 102 min (FOC) to 84 min (FOCUS), i.e., by 17.6% (Table 2). The latter was characterized by the shortest drying time among all experiments (mainly due to the lowest initial water content), although specific energy consumption was not the lowest (87.12 kJ/g H2O). The HPU treatment increased the drying rate by 47.3%, from 0.0131 g H2O/(g DM×min) (FOC) to 0.0185 g H2O/(g DM×min) (FOCUS). This observation points to the synergistic effect of ultrasound and osmotic dehydration, which indicates that this drying technique is highly promising. Cavitation probably disrupted the sugar layer on the surface of the material, which increased the material’s porosity and facilitated water evaporation.
In the remaining drying treatments (FCUS, FFCUS and FFOCUS), HPU did not induce a significant decrease in drying time or specific energy consumption (Table 2). On the contrary, in frozen fermented beetroot slices (FFC and FFCUS), the HPU treatment significantly prolonged drying time, from 129 min to 142 min (i.e., by 10.1%), and decreased the drying rate from 0.0755 to 0.0654 g H2O/(g DM×min) (i.e., by 13.4%). The curve in Figure 1C clearly indicates that HPU-assisted drying proceeded at a much slower rate from the very beginning of the process. The drying time of FC was also prolonged compared to FCUS, from 119 min to 127 min (i.e., by 6.7%). Ultrasonic vibration breaks down plant tissues and closes pores, which slows down the diffusion of water from inside the beetroot slice [Miano et al., 2021]. Convective drying of fermented (FC) and fermented and frozen/ thawed beetroot slices (FFC) without HPU was the least energy-consuming treatment (relative to the mass of removed water) due to the highest initial water content (8.80 and 9.85 g H2O/g DM, respectively) and average drying time.
In all fermented products not subjected to osmotic dehydration, fermentation significantly increased the drying rate compared to the raw samples. The drying rate of fermented beetroot slices (FC) was more than twice higher relative to raw beetroot slices (RC), reaching 0.0721 g H2O/(g DM×min) and 0.0318 g H2O/(g DM×min), respectively (Table 2). This significantly higher drying rate is consistent with previous observations made for fermented plant products such as, e.g., broccoli waste [Bas-Bellver et al., 2023]. Fermentation significantly reduced drying time and energy consumption compared to raw beetroot slices (Table 2). Drying time was 119 min for FC vs. 183 min for RC. Energy consumption was determined at 67.31 kJ/g H2O in FC, and it was much lower than in RC (102.52 kJ/g H2O). During fermentation, the structure of cell walls is disrupted through degradation of polysaccharides by enzymes (mainly cellulases and glycosidases), which led to the formation of a porous microstructure, increased water vapor permeability, and significantly accelerated the drying process [Bas-Bellver et al., 2023; Janiszewska-Turak et al., 2022].
A comparison of FC and FFC products revealed that freezing/ thawing did not shorten the drying time of fermented beetroots, whereas such an effect was reported for raw beetroots subjected to a freezing/thawing pre-treatment [Vallespir et al., 2018]. The above could be attributed to the fact that the internal structure of the beetroot matrix was significantly degraded during fermentation, which enhanced water diffusion and evaporation during drying and promoted structural breakdown after additional treatment, such as freezing/thawing, which somewhat inhibits these processes.
Osmotic dehydration alone significantly reduced the drying rate of fermented beetroot slices (FOC: 0.0131 g H2O/(g DM×min) vs. FC: 0.0318 g H2O/(g DM×min)). Moreover, this treatment also significantly shortened drying time (FOC: 102 min vs. FC: 119 min). The decrease in the drying rate resulted from the formation of a semi-permeable sugar layer on the surface of the material, which prevented water migration. In turn, drying time was shortened because some water had been removed from the material already during osmotic dehydration, which reduced the amount of energy needed for drying. As a result, the energy consumption associated with the drying process was higher in osmotically dehydrated products (FOC: 94.52 kJ/g H2O) than in fermented and not osmotically dehydrated beetroot slices (FC: 67.31 kJ/g H2O), but still lower than in raw beetroot slices (RC: 102.52 kJ/g H2O).
Microstructure and color of beetroot slices
The drying process with freezing and osmotic dehydration pretreatments had a significant impact on the color and structure of beetroot products (Figure 2, Table 3).
Table 3
Color parameters of raw beetroot slices and beetroot slices subjected to different treatments.
[i] The presented values are means ± standard errors. Values marked with identical letters in the same column for dried products do not differ significantly (p≥0.05). L*, lightness; a*, (+)redness/(−)greenness; b*, (+)yellowness/(−)blueness; ∆E1, total color difference relative to raw beetroot; ∆E2, total color difference relative to raw beetroot dried by hot air (RC); ∆E3, total color difference between the same samples dried with and without ultrasound; R, raw; F, fermented; O, osmotically dehydrated; FF, fermented and frozen/thawed; C, convective dried; CUS, convective dried with ultrasound assistance.
Figure 2
Microstructure of beetroot slices subjected to different treatments, 500× magnification. R, raw; F, fermented; O, osmotically dehydrated; FF, fermented and frozen/thawed; C, convective dried; CUS, convective dried with ultrasound assistance.
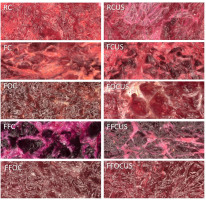
In Figure 2, dried raw beetroot slices (RC) present undisrupted shining cell walls. In beetroot slices dried with ultrasound (RCUS), bright zones were formed on the surface, denoting cell walls, which seems to be more ragged. The use of ultrasonic waves during convective drying led to the formation of microchannels, which slightly disrupted the structure of tissues, increased porosity and intercellular spaces [Rajewska & Mierzwa, 2017]. Ultrasound prevented sugar and water molecules from clumping and closing beetroot pores. The above improved the efficiency of water diffusion, accelerated the drying process, and improved the quality of the final product. Raw beetroot slices (RC) had an intense red-violet color and were characterized by the highest value of parameter a* (Table 3). Color parameters were similar (lack of statistically significant differences for parameters L* and a*) in beetroots dried with ultrasound assistance (RCUS) and only b* parameter was significantly lower and decreased from 3.1 to −0.1, which indicates a shift towards a more neutral or blue color. No significant changes were observed in parameter L*, which indicates that lightness remained fairly constant in the analyzed samples.
No sticking effect was observed in the fermented product (FC), where sugar content decreased during the fermentation process (Figure 2). The use of ultrasound induced minor changes in cellular structure of FCUS product and did not contribute to the formation of a sugar layer on the surface. Fermented beetroot slices (FC) were characterized by significantly lower values of parameters L* and a* than raw beetroots (RC), which indicates a color shift towards darker and less red (Table 3). Parameter a* decreased to 13.8 and parameter L* decreased to 20.1. The use of ultrasound in the fermented samples (FCUS) led to a further significant reduction in the values of these parameters compared to raw beetroot (RC), where L* reached 17.6, a* reached 10.6 and additionally b* reached −0.8. These changes indicate a significant color shift towards darker, greener and bluer shades, which could be attributed to pigment degradation during fermentation. A similar effect (decreasing of L* and b* parameters) was observed for apple slices by Mierzwa & Kowalski [2016].
The freezing/thawing treatment led to the formation of ice crystals capable of damaging cell walls. In Figure 2, structural damage with a large number of protrusions and cracks can be clearly observed in fermented and frozen/thawed (FF) products. The frozen/thawed sample had a normal cellular structure, which indicates that tissue integrity was preserved after this treatment. The fact that these structures were clearly visible could be related to higher porosity which facilitated water migration during drying. In the FFC product dried without ultrasound, clusters of cell walls were observed on the surface, probably because sugar was released from the inside of the material during the drying process. The moisture released during thawing contributed to the formation of a uniform sugar layer. In the samples dried with ultrasound (FFCUS), this process did not occur because residual sugars were evenly distributed throughout the material under the influence of ultrasound and were reabsorbed inside the material. Due to controlled distribution of water during freezing, glossy sugar residues and a more uniform structure were observed in the FFCUS samples. Even distribution of sugars and microstructural changes indicate that the synergistic effect of ultrasound and freezing/thawing treatments contributed to an improvement in the quality of dried beetroots.
Convective drying of fermented and frozen/thawed beetroot slices (FFC and FFCUS) produced darker and more purple beetroot products compared to dried beetroot without pre-treatment (RC) (Table 3). Additionally, the color of all osmotically dehydrated samples (FOC, FOCUS, FFOC and FFOCUS) was lighter than in the case of just frozen and fermented beetroot slices, which may be attributed to betanin leaching (the lowest FRAP and TPC – see Table 4) and sugars from the osmotic solution sticking to the surface of the slices. A significant decrease in the values of parameters a* and b* was noted in the fermented and frozen/thawed (FFC) product relative to the RC sample, which indicates a shift towards ruby red color. After the ultrasound treatment (FFCUS), the value of a* significantly increased to 11.6, which suggests that red color was partially restored, whereas the value of b* remained low (−1.1), indicating the presence of blue color.
Table 4
Total phenolic content (TPC), ferric reducing antioxidant power (FRAP), and sugar content (SC) of beetroot slices subjected to different treatments.
[i] The presented values are means ± standard errors. Values marked with identical letters in the same column do not differ significantly (p≥0.05). GAE, Gallic acid equivalent; TE, Trolox equivalent; DM, dry matter; R, raw; F, fermented; O, osmotically dehydrated; FF, fermented and frozen/thawed; C, convective dried; CUS, convective dried with ultrasound assistance.
During osmotic dehydration of fermented beetroot slices, water was removed from cells along the osmotic gradient. Beetroot slices subjected to osmotic dehydration and convective drying (FOC) had a compact and less porous structure (Figure 2). In addition, they had a higher sugar content (Table 4), and drying led to the formation of a glossy sugar layer on their surface. The ultrasound treatment increased the penetration of the osmotic sugar solution, highlighting pores and cell walls in the FOCUS samples. In these samples, ultrasound promoted the diffusion of sugar molecules inside beetroot tissues and increased sugar saturation. Ultrasound-assisted drying of osmotically dehydrated beetroot slices (FOCUS) induced additional changes, including increased porosity and the formation of micro-channels, which improved drying efficiency. The benefit was not as great as expected, but is somehow contrasting with results obtained by Mierzwa & Kowalski [2016], who dried previously osmotically dehydrated apple slices under HPU assistance. Drying osmotically dehydrated beetroot slices (FOC) decreased the value of parameter a* to 12.3 and increased the value of parameter b* to 4.5 compared to beetroot dried without pre-treatment (RC) (Table 3). The change in the color (ΔE2) of osmotically dehydrated and dried beetroot slices (FOC) was similar to the values reported by Calderón-Chiu et al. [2020]. These authors reported that ΔE reached 8.19 in osmotically dehydrated beetroots (5-mm thick slices, temperature of 30°C, 65% sugar content) dried at 85°C using the refractance window method. The ultrasound drying of osmotically dehydrated beetroot slices (FOCUS) induced the smallest total color difference (ΔE3 = 2.7) relative to the FOC sample (Table 3).
The structure of FFOC and FFOCUS products was similar to that of FOC samples (Figure 2). Additionally, the samples that were and were not subjected to ultrasound-assisted drying had an identical structure. These results indicate that in osmotically dehydrated samples, freezing caused pores to close after the freezing/thawing cycle. In these samples, sugars were dissolved by water absorption during thawing, which promoted the formation of a compact structure and could explain the difference relative to the FOCUS product. The values of color parameters were similar in FFOC and FFOCUS products, where a* was determined at 12.9 (FFOC) and 9.9 (FFOCUS), and b* was determined at 3.7 (FFOC) and 2.9 (FFOCUS), which indicated that the ultrasound treatment had a negligent impact on red and yellow hues of fermented, osmotically dehydrated, and frozen beetroot slices. However, the total color difference between these products was high (ΔE3 = 6.0).
Total phenolic content, antioxidant capacity, and sugar content of dried beetroot slices
The total phenolic content, antioxidant capacity, and sugar content of dried beetroot slices differed significantly across treatments (Table 4). The TPC and FRAP were highest in raw beetroot slices dried with ultrasound assistance (RCUS) and lowest in osmotically dehydrated products (FOC, FOCUS, FFOC, FFOCUS). The total phenolic content ranged from 1.45 to 9.05 mg GAE/g DM, FRAP ranged from 2.19 to 24.45 mg TE/g DM, and SC ranged from 2.40 to 47.20 g/100 g DM.
Sugar content was highest in raw beetroot slices subjected to convective drying (RC) and ultrasound-assisted convective drying (RCUS). The sugar content of these products was 47.20 and 44.40 g/100 g DM, respectively. The ultrasound treatment induced a minor decrease in sugar content, but the observed difference was not significant. Ultrasound could have influenced the microscopic distribution of sugars in beetroot tissues, but the overall sugar content remained high. Ultrasound-assisted drying of raw beetroot slices led to a significant increase in TPC and FRAP of the RCUS product relative to the sample dried without ultrasound (RC) (Table 4). The TPC increased from 7.73 to 9.05 mg GAE/g DM, and FRAP increased from 19.52 to 24.45 mg TE/g DM. The above increase probably resulted from shorter drying times (by up to 23%) (Table 2), which minimized the thermal degradation of phenolic compounds. Additionally, the cavitation effect generated by ultrasound disrupted the plant matrix, which facilitated the extraction of bioactive compounds and increased the antioxidant capacity of dried beetroots. This result is consistent with the literature data concerning drying raspberries and goldenberries [Ashtiani et al., 2022; Cakmak et al., 2021].
Ultrasound-assisted drying of fermented beetroot slices had a minor effect on TPC which remained fairly constant in FC (8.09 mg GAE/g DM) and FCUS (8.04 mg GAE/g DM) products (Table 4). However, FRAP increased from 16.13 to 18.10 mg TE/g DM. The sugar content of FC and FCUS products was determined at 12.40 and 10.80 g/100 g DM, respectively, and was significantly lower than in raw beetroots. Most probably, this difference can be attributed to fermentation during which sugars were used by bacteria as a substrate for the production of lactic acid [Montet et al., 2014]. Ultrasound-assisted drying had no significant effect on the sugar content of fermented beetroot slices, which suggests that the effect of fermentation outweighed the potential effect of the ultrasound treatment.
The freezing/thawing treatment before convective drying led to the degradation of phenolic compounds. As a result, TPC decreased from 8.09 mg GAE/g DM (FC) to 7.26 mg GAE/g DM (FFC), and FRAP decreased from 16.13 mg TE/g DM (FC) to 15.27 mg TE/g DM (FFC) (Table 4). These changes were most likely caused by freezing damage to beetroot tissues. The phase change of water from a liquid to a solid phase (ice) led to the breakdown of cell walls and the loss of bioactive compounds [Dalmau et al., 2019; Vallespir et al., 2019]. Ultrasound-assisted drying of frozen/thawed beetroot slices led to a further decrease in TPC and FRAP of the FFCUS product (by 18% and 15%, respectively) compared to the FFC sample (Table 4). The cavitation effect generated by the ultrasound treatment resulted in a greater loss of natural juice during drying, which decreased the TPC and FRAP. The lowest sugar content was noted in fermented and frozen/thawed beetroot slices that were or were not osmotically dehydrated (FFC, FFCUS, FFOC and FFOCUS). The sugar content of these products ranged from 2.40 to 4.60 g/100 g DM. The freezing/thawing treatment led to a significant loss of sugars during thawing [Screde, 1983]. It is worth noting that the ultrasound treatment promoted a further decrease in the sugar content of FFCUS product, probably because the sugar leaching process was intensified under the influence of ultrasonic cavitation.
Convective drying of osmotically dehydrated beetroot slices led to a significant decrease in TPC and FRAP of the FOC product compared to the FC sample (Table 4); TPC content was reduced by 82% (from 8.09 to 1.46 mg GAE/g DM), whereas FRAP decreased by 86% (from 16.13 to 2.19 mg TE/g DM). Most probably, the observed decrease was caused by water diffusion during osmotic dehydration when water-soluble phenolics were removed from beetroot tissues into the surrounding osmotic solution. Phenolic compounds were further degraded during convective drying, and TPC and FRAP were lowest in osmotically dehydrated samples (FOC, FOCUS, FFOC, FFOCUS). The ultrasound treatment had no significant effect on TPC and FRAP during the drying of fermented, frozen/thawed, and osmotically dehydrated beetroot slices. This may be due to considerable tissue deformation during freezing/thawing and osmotic dehydration, which reduced the impact of the ultrasound treatment during drying. Similar observations were made by other authors for beetroot and celery root [Nicetin et al., 2022; Staniszewska et al., 2024]. In the group of fermented and dried beetroot samples, sugar content was highest in FOC and FOCUS products (31.00 and 30.40 g/100 g DM, respectively). These products had the highest sugar content because sugar was adsorbed from the osmotic solution during osmotic dehydration [Corrêa et al., 2010]. This process promoted the accumulation of sugars in beetroot slices, which increased their sugar content. However, the sugar content of FFOC and FFOCUS products was several times lower because sugars were most probably removed during thawing. As a result, the final sugar content of FFOC and FFOCUS samples was determined at 4.60 and 4.40 g/100 g DM, respectively.
Fourier-transform infrared spectroscopy data of dried beetroot slices
The FTIR spectra of dried beetroot slices are shown in Figure 3. Different absorption bands of these spectra (3,293; 2,927; 1,736; 1,638; 1,421; 1,375; 1,330; 1,241; 1,104; 1,038; 988; 922; and 828 cm–1) are associated with chemical bonds characteristic of carbohydrates, alcohols, carboxylic acids, esters, alkenes, ketones, pectins, and other organic compounds present in beetroots. The 3,293 cm–1 band corresponds to the stretching vibrations of the hydroxyl group (–OH) which is present in water and alcohols. In beetroots, this band may indicate the presence of carbohydrates and water. The 2,927 cm–1 band indicates the presence of C–H bonds related to the stretching vibrations of alkyl groups (–CH3, –CH2–) [Singh et al., 2017; Xin et al., 2020]. The 1,736 cm–1 band corresponds to the stretching vibrations of the carbonyl bond (C=O) in esters and carboxylic acids, and it may be associated with the presence of hemicellulose, pectins, and other organic compounds in beetroots. The 1,638 cm–1 band can be attributed to the stretching vibrations of C=C bonds in alkenes and C=O bonds in amides and ketones, which may correspond to organic dyes such as betanin [Aztatzi-Rugerio et al., 2019]. The 1,421 cm–1 band corresponds to the bending vibrations of the methyl group (–CH3) and the carboxylate group (COO–); the 1,375 cm–1 band is associated with the bending vibrations of the methyl group (–CH3), whereas the 1,330 cm–1 band corresponds to the bending vibrations of the methoxy group (–OCH3) and the deformation vibrations of the nitro group (NO2). In turn, the 1,241 cm–1 band is attributed to the stretching vibrations of C–O bonds in esters and carboxylic acids, which may indicate the presence of pectins and other polysaccharides [Monsoor, 2005; Xin et al., 2020]. The 1,104 cm–1 band corresponds to the stretching vibrations of C–O–C bonds in ethers and glycosidic bonds in sugars, and the 1,038 cm–1 band corresponds to the stretching vibrations of C–O bonds in alcohol, which may be related to the presence of carbohydrates and cellulose [Xin et al., 2020]. The 988 cm–1 band corresponds to the stretching vibrations of C–H bonds in alkenes and the bending vibrations of the CH2 group in carbohydrates; the 922 cm–1 band corresponds to the stretching vibrations of C–O–C bonds in acid anhydrides and the deformation vibrations of alkene C–H bonds, whereas the 828 cm–1 band corresponds to the bending vibrations of C–H bonds in alkene groups and the deformation bonds of the aromatic group [Aztatzi-Rugerio et al., 2019].
Figure 3
Fourier-transform infrared spectra of beetroot slices subjected to different treatments. R, raw; F, fermented; O, osmotically dehydrated; FF, fermented and frozen/thawed; C, convective dried; CUS, convective dried with ultrasound assistance.
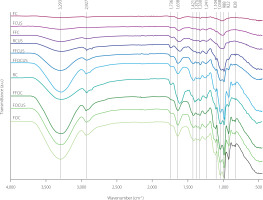
These results indicate that fermentation, freezing/thawing, osmotic dehydration, and convective drying with or without ultrasound treatment significantly influenced the intensity and position of absorption bands. The highest intensity of the bands corresponding to C=C multiple bonds was observed in raw beetroot slices subjected to convective drying. These results correlate with findings of Lohumi et al. [2017]. The bands at 1,638; 1,105; and 1,037 cm–1 were associated with changes in pigment content during ultrasound-assisted drying of raw beetroot slices. The ultrasound-assisted drying of raw and frozen/thawed beetroot slices subjected to osmotic dehydration led to the loss of pigments. Fermented beetroot slices subjected to ultrasound-assisted drying were characterized by a higher pigment content because betanin was stabilized by hydrogen cations formed during the fermentation process [Atav & Yurdakul, 2016; Volia et al., 2019]. Fermentation led to the degradation of sugars, which significantly reduced the intensity of the absorption bands associated with hydroxyl groups (3,293 cm–1).
The freezing/thawing process led to the formation of ice crystals inside cells, which increased the risk of damage to cell walls. The FTIR spectra of fermented and frozen/thawed (FF) product revealed clear structural damage. A higher content of deposited water in frozen samples increased the intensity of the bands corresponding to the hydroxyl group (3,293 cm–1). At the same time, the decrease in the intensity of the bands corresponding to C=C and C=O groups denoted the loss of pigments, as indicated by the lower values of parameters a* and b* (Table 3).
The absorption bands in the FTIR spectra of the osmotically dehydrated sample (FOC) had the highest intensity in the range of 3,700–2,800 cm–1, which corresponds to the hydroxyl group and is associated with higher sugar content. Higher band intensity was also associated with the content of the pigment produced during osmosis. The intensity of these bands was lower in dried fermented beetroot slices, which could be attributed to the lower content of water and the breakdown of carbohydrates during lactic acid fermentation [Nizamlioglu et al., 2022]. Band intensity was somewhat higher in frozen beetroot slices due to the higher content of water deposited during freezing [Zaritzky, 2010].
CONCLUSIONS
The study demonstrated that the high-power ultrasound treatment applied during convective drying exerted the most beneficial effect on raw beetroot slices by reducing drying time and energy consumption. The ultrasound treatment also significantly increased total phenolic content and the ferric reducing antioxidant power, and induced a negligible shift in color parameters toward blue (more purple than red). Considering the pretreatments, ultrasound-assisted drying shortened the drying times of fermented and osmotically dehydrated beetroot slices, increased the total phenolic content and the ferric reducing antioxidant power of this sample, and increased the ferric reducing antioxidant power of fermented beetroots. In the remaining cases (such as beetroots only fermented or fermented and frozen/thawed – both osmotically dehydrated and not), the ultrasound treatment did not induce significant beneficial changes in the drying process or the properties of the final product. Sometimes the changes were undesirable, such as significant prolongation of drying time in fermented and frozen/thawed beetroots, a decrease in total phenolic content with the associated reduction in the ferric reducing antioxidant power.
The combination of fermentation, freezing/thawing, and ultrasonication treatments led to severe structural damage, which increased porosity and promoted water migration during the drying of beetroot slices. Interestingly, osmotically dehydrated beetroots subjected to ultrasound-assisted drying had a more uniform structure with micro-channels, which increased drying efficiency. All analyzed treatments decreased the intensity of red color in dried beetroot slices. However, in some cases changes in the color of dried beetroots (some color parameters for FOCUS, FFOCUS) were unnoticeable, which can be deemed as desired. The observed changes in the values of color parameters L*, a*, and b* denote the extent to which these processes affected the appearance of the final product, which is an important consideration from the consumers’ point of view.
Fermentation alone shortened the convective drying time of beetroot slices and significantly decreased the sugar content in relation to drying beetroot without additional treatments. This can be of use in the production of dietetic snacks. Additionally, osmotic dehydration of fermented beetroot slices shortened convective drying time in relation to fermentation alone, which besides the energy savings can allow modifying the taste of the mentioned dietetic beetroot snacks. Subsequent freezing-thawing of fermented and osmotically dehydrated beetroots shortened convective drying time, but slightly prolonged convective drying of fermented beetroots. The above along with the results of antioxidant capacity leads to the conclusion that the production of fermented beetroot, followed by freezing and drying, can provide an interesting dietetic product at reduced costs.
In the future, other ultrasound parameters, such as power, exposure time and frequency, should be examined to optimize the ultrasound-assisted drying of raw (RCUS), fermented and osmotically dehydrated (FOCUS) beetroots. Ultrasound-assisted drying is not recommended for processing beetroots that are fermented only or frozen/thawed and fermented.

