ABBREVIATIONS
ABTS, 2-2′-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid); ATR-FTIR, Attenuated total reflectance-Fourier transform infrared spectroscopy; CUPRAC, Cupric ion reducing antioxidant capacity; DPPH, 2,2-Diphenyl-1-picrylhydrazyl; ORAC, Oxygen radical absorbance capacity.
INTRODUCTION
Truffles (Tuber spp.) are edible Ascomycota, hypogeous ectomycorrhizal funguses which live in symbiosis with the plant. What is classically named “truffle” consists in a developed fruiting body corresponding to hyphae aggregate. Truffles have been used as human food since the Bronze era [Shavit, 2014].
At least 180 truffle species are currently known worldwide [Bonito et al., 2010] although only 70 are undoubtedly established [Ceruti et al., 2003; Jeandroz et al., 2008]. Six truffle species are found and/or commercialised in France: Tuber aestivum, Tuber brumale, Tuber indicum, Tuber magnatum, Tuber melanosporum, and Tuber uncinatum. On the one hand, some truffle species are in great demand due to their special taste and aroma. These are mainly T. melanosporum, the most wanted and cultivated species, T. uncinatum and to a lesser extent T. magnatum (from Italy). On other hand, other species like T. aestivum, T. brumale and T. indicum, have an inferior flavour and are less sought after. However, distinguishing fruitbodies based on their morphology is not possible due to their similar appearance and colour. Therefore, fraud risks may arise by marketing low-value truffle species as high-value species. Thus, molecular methods for the identification of truffle species (DNA marker identification) have been developed since the 1980’s, especially for T. melanosporum and T. magnatum identification [Paolocci et al., 1997; Rubini et al., 1998; Séjalon-Delmas et al., 2000]. However, there are no data available concerning T. brumale DNA, and it has never been tried to discriminate up to 6 truffle species. To sum up, there is no approach today, allowing to discriminate the 6 Tuber species met in France.
Truffle chemical composition is already known [Yan et al., 2017]. Their cell wall contains chitin, chitosan, glucans, mannans, and proteins [Bowman & Free, 2006] sometimes associated with melanin mainly in the case of black truffles [Gessler et al., 2014]. They contain phenolics including flavonoids [Beara et al., 2014; Shah et al., 2020b] and fatty acids [Sancholle et al., 1988; Yan et al., 2017]. Some information about their mineral composition is also available in the Ciqual French food composition table (https://ciqual.anses.fr/). Surprisingly, identification approaches of Tuber species based on their composition variation have never been developed.
In addition to their composition, truffles have a number of interesting properties including antioxidant potential [Shah et al., 2020a; Tejedor-Calvo et al., 2021; Wu et al., 2021]. Indeed, antioxidants in food are beneficial for health with an impact at a molecular scale, inhibiting lipid oxidation for instance [Zehiroglu & Ozturk Sarikaya, 2019], and also their broader effects are supposed preventing putatively Alzheimer’s disease through, e.g., inhibition of acetylcholinesterase [Wilson et al., 2017]. Tejedor-Calvo et al. [2021] demonstrated 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity and/or ferric reducing antioxidant power (FRAP) and/or oxygen radical absorbance capacity (ORAC) of truffle species. The antioxidant capacity of truffles is therefore a potential way to discriminate truffle species.
Considering all these data together, it appears interesting to explore variations in truffle chemical composition, chromaticity and antioxidant capacity to discriminate truffle species. This study proposes to evaluate in this respect the six species of truffles found or sold in France including T. aestivum, T. brumale, T. indicum, T. magnatum, T. melanosporum, and T. uncinatum. Firstly, biophysical analysis of the truffles using attenuated total reflectance-Fourier transform infrared spectrometry (ATR-FTIR), chromaticity and atomic absorption spectrometry were carried out. Then, their antioxidant activity was evaluated using hydrogen atom transfer and single-electron transfer approaches. The detection of the authenticity of truffle species can be used in science to detect fraud.
MATERIALS AND METHODS
Biological materials
T. melanosporum was bought from “Truffefrance” (SAS Terroir Gourmand, Millau, France); T. magnatum from S.Z. Tartufisnc (Atessa, CH, Italy) and Tuber aestivum from La Rabasse De L’enclave (Valreas, France). Listed truffles were purchased in dehydrated slices form. T. brumale and T. uncinatum were purchased in frozen broken from La Rabasse De L’enclave (Valreas, France). T. indicum (Himalayan truffles) was purchased canned from Maison Borde (Saugues, France) and then was frozen. All frozen samples were lyophilised for 3 days. Each sample of dehydrated truffle was powdered using a grinder (IKA A11 classic, Staufen im Breisgau, Germany) for 30 s. These powders were stored in an oven at 40°C (a humidity-free environment) before analysis.
Chemicals
FeCl3, KCl, neocuproine and nitric acid were purchased from Sigma (Saint Louis, MO, USA). 2-2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), absolute ethanol, ammonium acetate, CaCl2, NaCl, CuCl2, CuSO4, gallic acid, MgSO4, potassium persulfate, and Trolox were purchased from Grosseron (Coueron, France).
ATR-FTIR spectrometry analysis
Reflectance spectra were acquired using a Bruker V70 interferometer (Bruker, Billerica, MA, USA) working under a dehydrated airflow in a reflectivity mode, with an ATR accessory containing a gold crystal. The measurements were performed for wave numbers from the middle of the infrared range (between 500 cm−1 and 4,500 cm−1). The instrumental resolution was about 1 cm−1, and measurements were averaged on 64 scans. Ten measures were done for each truffle species. ATR-FTIR measure is not a directly quantitative approach; hence, to present different spectra on a comparable scale, they were normalised by their standard deviation. To provide quantitative analyses, ratios between IR bands were established as used in the literature [Renouard et al., 2014; Rytwo et al., 2015].
Chromaticity analysis
Chromaticity analyses were conducted on a dehydrated truffle powder using Konica Minolta CR-400 colorimeter (Tokyo, Japan). Coordinates in the CIELab colour space were recorded, i.e., lightness (L*), redness/greenness (a*) and yellowness/blueness (b*). Ten measures were performed for each truffle species.
Mineral composition analysis
Dehydrated truffle samples were heated to redness in a muffle furnace at 700°C for 7 h. The ashes obtained were then solubilised in 1 to 5% nitric acid. The main ions found in living organisms: calcium, copper, iron, magnesium, potassium and sodium, were analysed from these solutions using an atomic absorption spectrometer AAnalyst 200 Perkin Elmer (Waltham, MA, USA). Quantification was performed based on the calibration curves (with R2>0.99) obtained using NaCl, CaCl2, KCl, FeCl3, CuSO4, and MgSO4. Five measurements were performed for each truffle species.
Extraction and quantification of phenolic compounds
Dried truffle powders (30 mg) were mixed with 1 mL of an ethanol/water (40/60, v/v) mixture. Samples were sonicated for 30 min at 40°C and 37 kHz (Elmasonic P30H ultrasonic bath, Singen, Germany); then centrifuged at 525×g for 10 min. Lastly, supernatants were collected for phenolic compound content and antioxidant capacity analyses. Five repetitions were conducted per species.
Total phenolic compound quantification was carried out using the Folin–Ciocalteu assay [Singleton & Rossi, 1965]. An aliquot of 50 μL of the supernatant (or 40% ethanol as control) was added to 800 μL of distilled water. Then, 50 μL of the Folin–Ciocalteu reagent were added, and the mixture was vortexed and left to stand for 5 min. A volume of 100 μL of 1 M Na2CO3 solution was then incorporated, and the mixture was incubated in the dark for 2 h. Finally, the absorbance of the solution was measured at 725 nm with a spectrophotometer microplate (FlexA-200, Allsheng, Hangzhou, China). A gallic acid calibration curve was plotted in the concentration range of 12.5–400 mg/L. Each measurement was performed in triplicate, and the total phenolic content was expressed as mg of gallic acid equivalent (GAE)/g dry weight (DW) of truffle powder.
Antioxidant capacity analysis
Two methods were used to evaluate the antioxidant capacity of truffles. Firstly, the ABTS•+ scavenging capacity was determined based on Re et al. [1999] method with few adaptations. The ABTS•+ was obtained from the reaction between the ABTS solution (7 mM in Milli-Q water) and the 2.45 mM potassium persulfate solution. The mixture was incubated in darkness at 25°C for 16 h. The ABTS•+ solution was diluted till its absorbance reached 0.7 at 734 nm. Then, 3 mL of the ABTS•+ solution was mixed with 100 μL of the truffle extract at 30°C for 10 min, and its absorbance was measured at 734 nm. All solutions were used no later than 24 h after preparation. The analyses were performed in triplicate, and the ABTS•+ scavenging capacity was expressed as μmol Trolox equivalent/g DW using a standard curve for Trolox with the concentration in the range of 31.25–500 μM (R2=0.9991).
The second method, the cupric ion reducing antioxidant capacity (CUPRAC) of truffles was determined with a protocol adapted from Apak et al. [2007]. An aliquot of 10 μL of the truffle extract was added to 190 μL of a 0.01 M copper (II) chloride solution. The copper (II) chloride solution consisted in a mixture of CuCl2×2H2O, ammonium acetate buffer (pH 7) and 7.5×10−3 M neocuproine (2,9-dimethyl-1,10-phenanthroline) diluted in ethanol, with the following proportions: 1:1:1, v/v/v). After 30 min rest, the absorbance was measured at 450 nm. The CUPRAC was expressed as μmol Trolox equivalent/g DW. Standard curve plotted for Trolox (31.25–1,000 μM, R2=0.9993) was used to calculate the results.
Statistical analysis
All data were presented in this study as the mean and standard deviation of ten replicates for ATR-FTIR and chromaticity analysis; five replicates for atomic absorption analysis, and three for CUPRAC, ABTS and Folin-Ciocalteu assays. Comparative statistical analysis of groups was performed using Student’s t-test. Statistical differences were considered to be significant at p<0.05. Graphical and statistical treatments were performed using Microsoft EXCEL 2010 software (Microsoft, Redmond, WA, USA). The principal component analysis (PCA) was performed using XLSTAT 2022.2.1 software (Addinsoft, Paris, France).
RESULTS AND DISCUSSION
Chemical characterization of truffles using ATR-FTIR spectroscopy
The ATR-FTIR measurement is not a directly quantitative approach. Considering one sample, ATR-FTIR analysis will provide spectra always presenting the same proportion between the IR bands but from one measure to another – the global spectrum intensity may change. Accordingly, quantitative analysis requires using ratios between IR bands. Then, to present different spectra on a comparable scale, they need to be normalised.
Zhao et al. [2006] demonstrated the infrared spectrometry ability to discriminate truffle species on 3 Chinese truffle species. On the other hand, in addition to being a simple, fast and economical biophysical approach, ATR-FTIR is known as a relevant tool in food fraud detection [Valand et al., 2019].
In Figure 1A, ATR-FTIR spectra of truffles of different species are presented as normalised by their respective standard deviation. First, the six species presented a global similar profile. Moreover, these profiles were similar to those obtained via the FTIR analysis by Zhao et al. [2006]. Four common massive absorbance areas were noteworthy in the truffles FTIR spectra (Figure 1A). The first area centered at 3,390 cm−1 corresponded to the stretching mode of hydroxyl bond (abundant in biological samples) [Grošev et al., 2001; Pappas et al., 2003]. The second area, between 3,000 and 2,800 cm−1, corresponded to fatty acid found in cell membranes with a band at about 2,925 cm−1 assigned to out-of-phase CH2 stretching and another at about 2,855 cm−1 assigned to in-phase CH3 stretching [Lin et al., 2004; Pappas et al., 2003]. The third area, between 1,800 and 1,500 cm−1, showed two main bands centered at about 1,650 and 1,560 cm−1 that are respectively associated to amide I and amide II [Payne & Veis, 1988] herein met in chitin and chitosan, which are two important fungal cell wall components. The last area, between 1,250 and 750 cm−1 and presenting an important maximum at about 1,042 cm−1, was assigned to C–O stretching in carbohydrate [Grošev et al., 2001; Pappas et al., 2003; Silva et al., 2001].
Figure 1
Normalised attenuated total reflectance-Fourier transform infrared (ATR-FTIR) spectra (A), chitin/chitosan ratio (1,659 cm–1/1,627 cm–1) (B), and β/α-glucan ratio (889 cm–1/850 cm–1) (C) of powders of Tuber species. Bars with different letters differ significantly one from another at p<0.05.
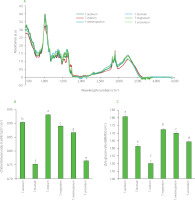
To discriminate truffle species by ATR-FTIR, it was necessary to look for the discriminating ratio linked to the truffle’s composition. Among all the fungal components for which specific IR bands are known (α- and β-glucan, aromatic compounds, chitin, chitosan, fatty acids, melanin and proteins), only two IR band ratios allow to statistically distinguish the six-truffle species: a chitin/chitosan ratio (1,659 cm−1/1,627 cm−1) and a β/α-glucan ratio (889 cm−1/850 cm−1). Dahmane et al. [2014] established that 1,659 cm−1 and 1,627 cm−1 IR bands corresponded respectively to chitin and chitosan from fungi cell wall. For the chitin/chitosan ratio, the ratio values ranged from 0.753 for T. brumale to 0.931 for T. indicum (Figure 1B). Concomitantly, Synytsya & Novak [2014] presented 889 cm−1 and 850 cm−1 as IR bands of β- and α-glucan respectively. For the β/α-glucan ratio, the ratio values ranged from 1.50 for T. indicum to 1.81 for T. aestivum (Figure 1C). Thus, both chitin/chitosan ratio and a β/α-glucan ratio allowed to perfectly distinguish the six-truffle species.
Truffles, like all fungi, are characterised by their virulence: their growth time, their resistance to other organisms, their ability to find and interact with their host etc. Indeed, truffles are symbiotic fungi associated with a plant, but competition can exist between soil organisms hoping to interact with a plant (for instance T. brumale is able to compete with T. melanosporum) [Merényi et al., 2016]. The ATR-FTIR results obtained in this study reflect the difference in virulence of each of the six-truffle species through chitin/chitosan ratio because the chitosan (obtained from chitin) is physiologically involved in the fungal virulence and evasion of host immune responses [Mouyna et al., 2020].
Mineral composition of truffles
Atomic absorption analysis was performed to quantify calcium, copper, iron, magnesium, potassium and sodium, that are among the most frequently encountered ions in living organisms. Results indicate (Table 1) that only magnesium and calcium contents allowed T. aestivum, T. brumale, T. indicum, T. magnatum, T. melanosporum, and T. uncinatum discrimination.
Table 1
Mineral element content of Tuber aestivum, Tuber brumale, Tuber indicum, Tuber magnatum, Tuber melanosporum, and Tuber uncinatum (mg/g dry weight) analysed by atomic absorption.
No detailed data are available on the mineral ion composition of the different truffle species; but, according to the Ciqual French food composition tables (https://ciqual.anses.fr/) black truffles (without further species specification) show comparable calcium, copper, iron and sodium contents to our assays, but the magnesium and potassium contents presented therein (2.38 and 44.7 mg/g DW, respectively) were much higher than in our study (0.91 and 19.81 mg/g DW), potentially because the truffle species studied were different. Additionally, the https://www.nutritionvalue.org/ website indicates (without specifying the truffle species) similar values (i.e., included in our range of values) to the data obtained in this study (Table 1) for all the mineral elements tested.
The ionic analysis confirms virulence variation between truffle species through magnesium and calcium contents since these ions are directly involved in fungal virulence. Lange & Peiter [2020] reviewed fungal virulence modulation to calcium links notably to Ca2+ ATPase; and Suo et al. [2018] demonstrated magnesium ability to regulate fungal virulence especially using a magnesium transporter named Mgt2, a transporter associated to melanin synthesis.
Chromaticity coordinates of truffle colour
The colour analysis is an interesting approach for food characterisation [Pathare et al., 2013]. Herein, the colour analysis of truffle allowed to discriminate T. aestivum, T. brumale, T. indicum, T. magnatum, T. melanosporum, and T. uncinatum (Table 2). The values of L* ranged from 20.56 to 36.35, the values of a* ranged from 1.62 to 4.23 and those of b* ranged from 2.78 to 12.9. The L*, a* and b* values different statistically significant (p<0.05) between the six analysed species. The L* value is the most intuitive to use since the colour of truffles oscillates between black and white. Indeed, the L* values allowed to clearly discriminate between the six truffle species with a high value for T. aestivum and T. magnatum considered as white truffle species compared to the 4 other species considered as black truffle species.
Table 2
Colour coordinates of Tuber aestivum, Tuber brumale, Tuber indicum, Tuber magnatum, Tuber melanosporum, and Tuber uncinatum powders.
The chromaticity analysis also shows virulence involvement in truffle species discrimination since the melanin (the truffle black pigment), which we have been able to evaluate using L* values, enhances the virulence in some fungi [Nosanchuk et al., 2015].
Antioxidant capacity and total phenolic content of truffles
Antioxidants can neutralise reactive oxygen species using different mechanisms, which has led to the development of different methods to assess the antioxidant activity of a molecule. Some analytical methods are based on single-electron transfer reaction, such as CUPRAC; others are based on the hydrogen atom transfer reaction, such as ORAC, and still others can use both modes of action, as ABTS or DPPH assay [Apak et al., 2016].
In this study, the CUPRAC and ABTS assays were used, and results of antioxidant capacity determination of dried truffle powders are shown in Figure 2 A & B. The CUPRAC was determined for all truffle species, highlighting that they contained antioxidants involved in the single-electron transfer process. ABTS assay results present a significant gain compared to the CUPRAC measures for all Tuber species. The only reference data available on truffles antioxidant capacity in the literature were obtained by Tejedor-Calvo et al. [2021]. Unlike our study where the results are expressed in relation to dry matter, these authors expressed the results in relation to fresh matter. In addition, the measurement of the antioxidant capacity of truffles was carried out using different techniques but with similar principles. However, the information obtained is similar. Indeed, the values of the tests based on one approach (CUPRAC here for single-electron transfer reaction, ORAC in the publication for hydrogen atom transfer reaction) were lower than those from the tests involving both approaches (ABTS assay here, DPPH assay in the cited publication), which confirms that these two processes were involved in the antioxidant activity of the truffle extracts. In addition, we observed (Figure 2A) negligible CUPRAC activity compared to ABTS activity (Figure 2B), indicating that antioxidant activity was mainly due to the hydrogen atom transfer reaction. This is confirmed by Tejedor-Calvo et al. [2021] who reported ORAC almost as high as DPPH radical scavenging activity for the truffle species studied.
Figure 2
Cupric ion reducing antioxidant capacity (CUPRAC) (A), ABTS•+ scavenging capacity (B), total phenolic content (C) of powders of dried Tuber species. Bars with different letters differ significantly one from another at p<0.05. DW, dry weight; GAE, gallic acid equivalent.
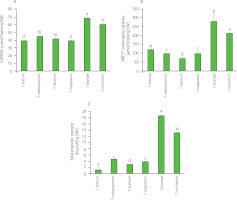
Focusing on truffle species discrimination, ABTS•+ scavenging capacity of truffles allowed to distinguish the different species (Figure 2B) except T. brumale and T. uncinatum. However, CUPRAC allowed to discriminate those two species, T. brumale and T. uncinatum. Moreover, ABTS•+ scavenging capacity of truffles did not allow to distinguish T. melanosporum and T. magnatum but it should be noted that it is difficult to confuse these both species since T. melanosporum was completely black while T. magnatum was white, as indicated by significant differences found in L* values between these species (Table 2).
The molecules directly associated with antioxidant activity are often phenolics [Bešlo et al., 2023]. For this reason, the total phenolic content of truffles was evaluated as a species discrimination approach. It ranged from 1.00 to 18.42 mg GAE/g DW (Figure 2C). Tejedor-Calvo et al. [2021] reported total phenolic content of different truffle species between 0.22 and 2.9 mg GAE/g FW. The proportion of water in different species of truffles is probably not the same; hence, it is difficult to compare our results with cited literature data expressed on a fresh matter basis [Tejedor-Calvo et al., 2021]. Nevertheless, water representing 75% of the truffle (https://ciqual.anses.fr/) and our phenolic compound assays showing amounts 5 to 6 times higher than those reported by Tejedor-Calvo et al. [2021] suggest that our extraction was effective. Piatti et al. [2024] found that the total phenolic content in T. magnatum was 2.41 mg GAE/g DW. This value was lower than that determined in our study for this species (3.69 mg GAE/g DW).
The differences in the total phenolic content between species were comparable to these in ABTS•+ scavenging capacity (Figure 2), allowing do discriminate all truffle species except T. melanosporum and T. magnatum.
Spiteller [2015] explains that mycelial cultures and fruiting bodies (e.g., truffles sold for food consumption) contain fungicidal phenolic compounds constituting a chemical defense. In other words, some truffle phenolic compound inhibits the development of other fungi, avoiding competition and favouring de facto the truffle development, the truffle virulence. Thus, the truffle phenolic compound content variation is associated to differences in truffle virulence capacities.
Determination of correlation between variables: a principal component analysis
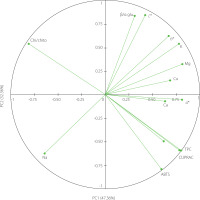
In order to determine the correlation between the discriminate variables studied, a principal component analysis was performed. The principal component 1 (PC1) explained 47.36% of the total variance, while the principal component 2 (PC2) was able to explain 32.36% of the variance, totalizing 79.72% (Figure 3). The PCA interpretation showed a strong correlation between the total phenolic content and CUPRAC, which is not surprising as phenolic compounds are significant antioxidants [Bešlo et al., 2023]. Furthermore, there was also a strong negative correlation between the total phenolic content (and CUPRAC) and the ATR-FTIR chitin/chitosan ratio. To explain it, it needs to be first reminded that chitosan is obtained by deacetylation of chitin. Then, knowing that (some) phenolics are capable to act on gene expression using epigenetic mechanisms [Číž et al., 2020] and that acetylation/deacetylation process can be regulated epigenetically [Liu et al., 2023], we can hypothesise that the increased production of phenolic compounds in Tuber regulates the conversion of chitin to chitosan through epigenetic mechanisms.
Figure 3
Plot of principal component analysis (PCA) on the set of factors that differentiate truffle species. ABTS, ABTS•+ scavenging capacity; β/α gluc., β/α-glucan ratio (889 cm–1/850 cm–1); Ca, calcium; Chi/chito, chitin/chitosan ratio (1,659 cm–1/1,627 cm–1); Cu, copper; CUPRAC, cupric ion reducing antioxidant capacity; Fe, iron; K, potassium; Mg, magnesium; Na, sodium; TPC, total phenolic content.
CONCLUSIONS
This study highlights the possibility of using ATR-FTIR, chromaticity and atomic absorption analyses in truffles species discrimination. Indeed, chitin/chitosan and β/α-glucans ratios determined by ATR-FTIR, colour coordinates and lightness as well as magnesium and calcium contents can help discriminate T. aestivum, T. brumale, T. indicum, T. magnatum, T. melanosporum, and T. uncinatum. Also, the ABTS•+ scavenging capacity and CUPRAC as well as the total phenolic content allowed to discriminate near than these six truffle species. Only T. melanosporum and T. magnatum could not be distinguished using antioxidant capacity measures. The potential of biophysical approaches, antioxidant capacity and/or total phenolic content analysis in the discrimination of truffle species has been highlighted in this study demonstrating direct or combined applications in food science such as in fraud detection.

