INTRODUCTION
The colloidal structure of ice cream has recently been an object of research interest for the global audience. It is known that the final physicochemical structure of ice cream may be regarded as a four-phase system, which includes air bubbles, fat globules, ice crystals and an unfrozen serum phase. In this unfrozen serum phase, there exist the unadsorbed casein micelles in a suspension of sugars, salts, whey proteins and high molecular weight polysaccharides. The structure of ice cream characterizes its complexity as a result of several stages of manufacturing such as pasteurisation, homogenisation, maturation, freezing and hardening. The colloidal aspects of ice cream, such as interactions between protein and emulsifier, the partial coalescence of fat or interactions between protein and partially coalesced fat, allow for a better understanding of this unique system [Gelin et al., 1994; Goff, 1997, 2002; Goff & Hartel, 2013; Konstantas et al., 2019].
During the manufacturing process, the ice cream mix emulsion is foamed, the dispersed phase of air bubbles is formed, and frozen to obtain another dispersed phase of ice crystals. The ice cream mix contains fat droplets, coated with a protein-emulsifier layer as a dispersed phase. The ice cream mix can also be classified as emulsion-filled gel, in which macromolecular gel contains dispersed fat particles (fillers). Therefore, the structure and rheological properties of the ice cream mix can be affected as a result of distinctive interactions of the filler gel matrix [Chen et al., 2019; Daw & Hartel, 2015; Goff, 1997; Innocente et al., 2009; Voronin et al., 2020].
Not only is the formation of the ice phase significant but stability is also equally crucial in the structure of ice cream. Desirable stability includes no changes in the size distribution or spatial arrangement of droplets. Stability plays a crucial role in controlling the shelf-life of ice cream or its texture and mouthfeel during consumption. It may be understood as the ability to resist undesirable changes in physicochemical properties. Loss of stability may occur due to various processes, such as creaming, flocculation, coalescence, partial coalescence, Ostwald ripening or phase inversion [Cheng et al., 2015; Dickinson, 1994, 2010; Pal, 2019]. The initial stability of emulsion in the ice cream mix is obtained by simultaneous adsorption of proteins and emulsifiers during the homogenisation process. Subsequently, during the maturation process, changes in the physical state occur in the liquid phase, not only in the emulsified fat but also in the adsorbed layer of mono- and diglycerides. Additionally other changes take place during maturation except for the mentioned process, such as the hydration of milk proteins and stabilisers, the crystallization of fat globules or the rearrangement of the surfactant membrane. They all contribute to a smoother texture and consequently better quality of ice cream [Gelin et al., 1994; Goff, 1997; Mendez-Velasco & Goff, 2012; Segall & Goff, 2002b].
Furthermore, the stability of emulsion in the ice cream mix may be considered through the prism of stabiliser addition. Despite the increasing viscosity, one of the main functions of stabilisers is to stabilise the emulsion and prevent separation [Akbari et al., 2019; Lomolino et al., 2020; Pintor & Totosaus, 2012; Seisun, 2010]. An example of such stabilisers is carrageenan that has been proved effective in preserving quiescent stability/shear instability in the emulsion with a low protein surface concentration [Segall & Goff, 2002a]. This happens as the migration of the milk protein from the solution to the oil-water interface in the emulsion is inhibited by carrageenans. Due to specific interaction with the milk proteins, carrageenans are employed in milk ice cream production. The favourable interactions between carrageenan and milk proteins depend on the number of sulphate groups in carrageenan structure and environmental conditions [Pintor & Totosaus, 2012]. ι-Carrageenan is one of the three forms of carrageenan (next to κ- and λ-carrageenan) that exhibits the specific ability to form a soft elastic gel in the presence of calcium ions, and stands out through other forms owing to the fact that it can form intra-molecular bonds between sulphate groups of anhydro-d-galactose and d-galactose via calcium as a divalent cation [Kiran-Yildrim et al., 2021; Thrimawithana et al., 2010]. Additionally, there are pieces of evidence that hydrolysates of carrageenan have a better ability to stabilize ice cream than native carrageenan. For instance, in the research by Kamińska-Dwórznicka et al. [2015] or Kot et al. [2022], it was noticed that hydrolysates of κ- and ι-carrageenan had more favourable effect on ice recrystallization inhibition (IRI) then κ-carrageenan in ice cream and ι-carrageenan in model solutions of ice cream, respectively.
This work focused on the influence of ι-carrageenan and its hydrolysates on the physical properties, such as stability, particle size distribution, consistency index and the flow behavior index, of milk ice cream mixes. Knowledge concerning these stabilisers in such products before freezing is lacking. Moreover, our previous research, in which the addition of ι-carrageenan and its acid and enzymatic hydrolysates allowed achieving desired size of crystals in a model milk solution, gave rise to the idea of using such stabilisers in this work [Kot et al., 2022]. This research might provide a valuable insight into the interactions between hydrocolloids and milk ice cream mixes.
MATERIALS AND METHODS
Materials
Pure ι-carrageenan powder was obtained from Sigma-Aldrich (St. Louis, MO USA). β-Galactosidase (1,000 U/mg, from Escherichia coli) and lactase (min. activity 5,200 NLU/g) for ι-carrageenan hydrolysis were purchased from Sigma-Aldrich and Serowar s.c. (Szczecin, Poland), respectively. The ingredients used to prepare the ice cream mixes were acquired from Mlekovita, Wysokie Mazowieckie, Poland (milk 0.5%, skimmed milk in powder); Orafti BENEO, Tienen, Belgium (inulin); Diamant, Poznań, Poland (white sugar); and Fooding Shanghai, Shanghai, China (emulsifier E471, locust bean gum (LBG), xanthan gum).
The preparation of hydrolysates of ι-carrageenan
The hydrolysis of ι-carrageenan was carried out according to the procedure described in our previous paper [Kot et al., 2022]. Briefly, ι-carrageenan was dissolved in distilled water heated up to 40°C to obtain a 0.4 mg/mL solution. The enzymatic hydrolysis was carried out using β-galactosidase for 72 h, at 37°C and a ratio of enzyme to ι-carrageenan solution of 1:1,000 (v/v) or using lactase for 24 h, at 5°C and enzyme to substrate ratio of 1:250 (v/v). For both hydrolyses, the reaction was stopped by neutralisation at 48°C for 5 min. To perform acid hydrolysis of ι-carrageenan, the substrate was dissolved in 0.1 M hydrochloric acid solution (pH 3). The solution was heated at 60°C for 3 h and then neutralised. All hydrolysates were stored frozen at –18° and thawed just before analysis. To characterise the hydrolysates, their molecular weight was determined by size-exclusion chromatography (SEC) according to the procedure described by Kamińska-Dwórznicka et al. [2015] using the Shimadzu high-performance liquid chromatography system consisting of a RID-10A detector, an LC-20 CE pump, a CTO-20A heater (Shimadzu, Kyoto, Japan) and equipped with a PolySep-GFC-P Linear column (300 mm x 7.8 mm, Phenomenex, Torrance, CA, USA). The molecular weight of samples after the hydrolysis by β-galactosidase ranged from 3.20×106 to 3.80×106 Da; that of hydrolysates obtained by commercial lactase from 3.50×106 to 3.60×106 Da, and that of acid hydrolysates from 1.48×106 to 1.94×106 Da.
The preparation of ice cream mixes
The ingredients of the ice cream mixes are presented in Table 1. The control sample (C) was prepared without stabilisers. The ice cream mix with the combination of ι-carrageenan, LBG and xanthan gum was coded as I. In turn, samples A, B and L instead of ι-carrageenan contained its acid hydrolysate or hydrolysates obtained using β-galactosidase and commercial lactase, respectively. All components were mixed using a Bosch Maxo -Mixx 750W blender (Bosch, Gerlingen, Germany). The next step involved pasteurization conducted in a Vorwerk thermomixer (Vorwerk, Wuppertal, Germany), at 85°C within 1.5 min. After this process, the mixtures were cooled to 25°C. Finally, the obtained ice cream mixes were matured for 24 h at 4°C in a refrigerator (Whirlpool, Warszawa, Poland). Two liters of ice cream mix were prepared for each experimental variant in duplicate.
Table 1
The composition (%) of ice cream mixes without stabilisers (control, C), with ι-carrageenan (I) and with hydrolysates obtained by acid (A), β-galactosidase (B) and commercial lactase (L) treatment of ι-carrageenan.
Stability analysis of ice cream mixes
The analysis of the stability of ice cream mixes was performed using a Turbiscan Lab Expert device (Formulation SA, Toulouse, France). The Turbisoft 2.0.0.33 software was used to evidence the date of backscattering (BS) and calculate the Turbiscan stability index (TSI). The ice cream mixes were analysed before and after maturation in three replications.
The analysis of particle size distribution of ice cream mixes
The particle size distribution of the ice cream mixes before and after maturation was measured by laser diffraction using a Cilas 1190 analyser (Cilas, Orléans, France). Few drops of emulsion of milk ice cream mixes were suspended in water at an obscuration of 10%. The results were presented as the median diameter (D50) and as diagrams of particle size distribution. Analyses of all samples were performed in three replications.
The rheological analysis of ice cream mixes
The rheological properties of the milk ice cream mixes were examined using the Haake Mars 40 rheometer (Thermo Scientific Inc., Karlsruhe, Germany) in a rotational mode within a shear rate of 0–100 s−1 at a constant temperature of 25°C. The analyses were performed in triplicate. The apparent viscosity (ηapp) curve as a function of shear rate (ẏ) in the semi-logarithmic scale (the flow curves) was plotted by the RheoWin v.4.86. Job Manager (Thermo Scientific). Based on the obtained results, only Ostwald de Waele model (1) was used to describe the flow curves and to determine the flow behaviour index.
where: K is the consistency index (Pa×sn), ẏ is the shear rate (s−1), and n is the flow behavior index (dimensionless).The adequacy of fitted model was estimated using regression analysis which delivered the correlation coefficient (R).
Characterization of the ice cream mix emulsion using confocal laser scanning microscopy (CLSM)
The microstructure of the emulsion of the ice cream mix (before and after the maturation step) was analysed using a confocal laser scanning microscope FLUOVIEW FV300 (Olympus, Tokyo, Japan), according to the method described by Ahn et al. [2022]. The fluorescence dye – Nile red – was used to label lipids in the samples. An aliquot of 200xL of the emulsion for all ice cream mixes and 3 μL of the Nile red solution (1 μg/mL) were mixed. Then, the samples were stained on glass slides, covered with a coverslip and observed at excitation and emission wavelengths of 630 and 660 nm, respectively. From every sample of ice cream mix, 6 photos were made and only representative ones were chosen for to results.
Statistical analysis
The results were expressed as a mean with standard deviations. The analysis of variance (ANOVA) was performed for the TSI, the D50 and data from rheological properties. The analysis was performed using STATISTICA 13.3 software (Statsoft Polska, Kraków, Poland) with test significance at α=0.05. The differences between homogenous groups were assessed using the Tukey’s honestly significant difference (HSD) test.
RESULTS AND DISCUSSION
The stability of ice cream mixes
The stability of milk ice cream mixes was scrutinised with the turbidimetric method, and respective results are presented as the TSI in Table 2 and as backscattering profile in Figure 1 (for control mix and mix with acid hydrolysate of ι-carrageenan) and in Figures S1–S3 in Supplementary Materials (for other ice cream mixes). The addition of stabilisers (ι-carrageenan and its hydrolysates) contributed to changes in the stability of milk ice cream mixes. The TSI ranged from 1.9 to 2.8. The lowest (p<0.05) value was noted for the sample with the addition of acid hydrolysate of ι-carrageenan (A). Moreover, it was noticed that there was no significant difference (p≥0.05) in the TSI of ice cream mixes with enzymatic hydrolysates of ι-carrageenan (B, after β-galactosidase treatment and L, after lactase treatment) and control sample without stabilisers (C). Nonetheless, the most striking observation was that the addition of ι-carrageenan contributed to the highest TSI value, which indicates slightly worse stability of the mix with ι-carrageenan compared to the other samples. Based on TSI, it may be stated that only the addition of acid hydrolysate of ι-carrageenan (A) contributed to significant improvement in the stability of ice cream mixes compared to the control sample. In research by Seo & Oh [2022], the TSI of ice cream mixes stabilized by the κ-carrageenan/milk protein isolate or Maillard conjugate derived from the reaction of the κ-carrageenan/milk protein isolate during 120 h of storage was less than 1.8.
Table 2
The results of physical properties analysis of milk ice cream mixes with stabilisers before and after maturation.
[i] Values are presented as mean ± standard deviations. Different superscript letters in column represent significant differences in the means (p<0.05). C, control ice cream mix (without stabilisers); I, ice cream mix with ι-carrageenan, locust bean gum (LBG) and xanthan gum; A, B and L, ice cream mixes with hydrolysates obtained by acid, β-galactosidase and commercial lactase treatment of ι-carrageenan, respectively, LBG and xanthan gum; TSI, Turbiscan stability index, D50, median diameter, K, consistency index (Pa×sn), n, flow behaviour index; R, correlation coefficient.
Figure 1
Backscattering (BS) profile of ice cream mixes without stabilisers (control sample) (A) and stabilised by the combination of acid hydrolysate of ι-carrageenan, locust bean gum and xanthan gum (B) before and after maturation. The dark green line presents measurement before maturation and the light green line presents measurement after maturation.

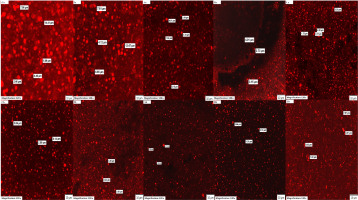
The backscattering profiles (Figure 1, Figures S1–S3) showed that during maturation time, two phenomena occurred in the milk ice cream mixes. Firstly, it was noticed that the same type of destabilization, such as coalescence/flocculation, occurred in the control sample (C) and in the sample with the addition of ι-carrageenan (I) and that the intensity of backscattering was changing across the whole height of the vial. Additionally, observations of CLSM images of these samples before and after maturation (Figure 2) confirmed destabilisation of fat in mixes, as indicated by agglomerates visible in images Ia, Ib (the sample with the ι-carrageenan) and Ca, Cb (the control sample). Furthermore, except for coalescence/flocculation, also sedimentation occurred in the milk ice cream mixes with the addition of acid hydrolysates of ι-carrageenan (Figure 1); as evidenced by significant changes in the intensity of backscattering on the top and bottom and also slightly in the middle of the vial. These processes of destabilisation may take place simultaneously (droplets coalescing during sedimentation) or one by one (for instance, firstly small droplets grow by coalescence before sedimenting). The separation in ice cream mix with ι-carrageenan hydrolysate (β-galactosidase treatment) before maturation was captured in image Ba (Figure 2). The order of this process may depend on the droplet diameter, dispersed phase concentration or viscosity [Frising et al., 2008]. What should be highlighted here is the fact that after the maturation, the median diameter (D50) for samples with the addition of ι-carrageenan or enzymatic hydrolysates decreased (Table 2). It is common knowledge that coalescence or flocculation occurs when the particle size increases not decreases [Goff et al., 1989]. However, as mentioned above, the agglomerates of fat of ice cream mixes were visible in CLSM images (Figure 2). In the research by Cheng et al. [2015], flocculation occurred despite a decrease in the average particle size after the maturation of ice cream mixes with different polysaccharide contents. In our research, despite the content of stabilisers and emulsifiers contributing to a decrease in particle size, the change was not significant enough to inhibit the mentioned type of destabilisation.
Figure 2
Confocal laser scanning microscopy images of ice cream mixes before (a) and after (b) maturation. C, control ice cream mix (without stabilizers); I, ice cream mix with ι-carrageenan, locust bean gum (LBG) and xanthan gum; A, B and L, ice cream mixes with hydrolysates obtained by acid, β-galactosidase and commercial lactase treatment of ι-carrageenan, respectively, LBG and xanthan gum.
It was proven that a certain amount of fat destabilisation is covetable in frozen products, such as ice cream [Berger & White, 1971; Goff et al., 1989; Koxhlot et al., 2001, Liu et al., 2022]. As a result of a combination of sheer forces and ice crystallisation during the freezing process, fat globules are mechanically damaged and agglomeration or coalescence takes place. Consequently, such desirable destabilisation contributes to dry appearance, slow meltdown, good shape retention and finally a firmer texture. Albeit, the excessive coalescence of fat may be associated with poor whipping properties or a buttery texture. Furthermore, it is known that the ice cream mix is not only an example of dairy emulsion but also a foamed dairy emulsion [Stanley et al., 1996]. It means that the stability of such products is connected with the presence of air bubbles. In such cases, preventing air bubbles to grow in size or adjacent to coalescing should be considered in the whole conception of the stability of ice cream mixes. Additionally, Liu et al. [2022], proved that the higher the amount of fat aggregates in the serum phase, the greater the possibility of a 3D destabilization network formation in ice cream. In our research, destabilisation occurred in ice cream mixes; thus, it may be concluded that fat destabilisation at this step of production could produce conditions for the formation of more favourable and uniform ice crystal structures while freezing. Finally, in the present study, the ice cream mixes remained stables despite TSI value.
The particle sizes of ice cream mixes
The median diameter (D50) of particles of milk ice cream mixes before and after maturation was presented in Table 2, and the particle size distribution in Figure 3. Before maturation, the D50 ranged from 17.56 to 45.50 μm. The lowest (p<0.05) value of this parameter was noted for the control sample without stabilisers (C), whereas the highest one – for the ice cream mix with the addition of acid hydrolysate of ι-carrageenan (A); however, the values determined for the mixes with ι-carrageenan and with its hydrolysate obtained using lactase did not differ significantly (p≥0.05) from that of sample A. Overall, before the maturation, the addition of stabilisers contributed to the increase in the median diameter of ice cream mix particles. After maturation, the D50 ranged from 23.24 to 46.73 μm. The tendency of the variation of this parameter was the same as before maturation. In the control sample (C), the median diameter was at 23.34 μm and in the sample with the addition of acid hydrolysate of ι-carrageenan (A) it reached almost 47 μm. Nonetheless, some changes occurred compared to the values before maturation. The decrease in the size of particles was observed in the ice cream mixes with the addition of enzymatic hydrolysates of ι-carrageenan (B and L) and in the sample with ι-carrageenan (I). On the other hand, a slight increase of D50 was noticed in the mix with the acid hydrolysates of ι-carrageenan (A) and the control sample (C). As a result of maturation, the greatest reduction of median diameter (by almost 6 μm) was observed for the sample with the ι-carrageenan hydrolysate obtained using lactase (Table 2). Such changes are desirable to obtain the expected quality of the final product, especially in the context of the formation of ice crystals. In our previous study, vegan ice cream mixes with the same stabilisers were analysed [Kot et al., 2021]. Similar results were noticed, i.e., the D50 of ice cream mixes decreased as a result of maturation, and only in the control sample was an increase observed. On the other hand, the size of the particles was smaller than in the present paper; the range of median diameter after maturation was from 17.23 to 28.50 μm. Overall, the reason for the large size of particles in ice cream mixes may be the addition of an emulsifier to the recipe. Based on the research by Liu et al. [2022], the fat aggregate size and also the fat aggregate percentage increased with the increased amount of the added emulsifier, such as Tweet 80, P4780 and whey protein isolate, to ice cream mixes. In the sample with the addition of 0.25% of emulsifier, the size of fat aggregates was from 1.6 to 66.3 μm. Bolliger et al. [2000] also discussed using emulsifiers in ice cream mixes and reported that the increasing amount of emulsifier contributed to the increased fat globule size or aggregate size of fat globules. Furthermore, in the samples without emulsifiers, no stable agglomerates were formed, while the only stable agglomerates were present in ice cream mixes with the addition of this ingredient. Alternatively, Alvarez et al. [2005] showed that maturation did not affect the size distribution of fat globules. The authors attributed it to a result of no noticeable destabilisation of emulsions during maturation due to the fact that the changes in the structure of emulsion, such as rearrangement of fat globule membranes, contributed to the lesser stability during maturation.
Figure 3
Particle size distribution of ice cream mixes before (A) and after (B) maturation. C, control ice cream mix (without stabilizers); I, ice cream mix with ι-carrageenan, locust bean gum (LBG) and xanthan gum; A, B and L, ice cream mixes with hydrolysates obtained by acid, β-galactosidase and commercial lactase treatment of ι-carrageenan, respectively, LBG and xanthan gum.
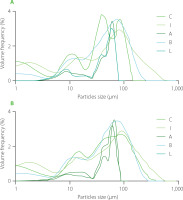
It is known that the changes in the structure of ice cream mixes, such as the arrangement of fat droplets, affect their texture as well as the melting stability of the final product [Mendez-Velasco & Goff, 2012]. The uniform distribution of droplets may be the reason for kinetic instabilities such as creaming or sedimentation [Pal, 2019]. Therefore, the particle size distribution of ice cream mixes was analysed and the results are shown in Figure 2. Considering particle size distribution of the samples before maturation, four peaks were observed for the control sample (with particle size ranges of 0–2, 7–30, 50–80 and 100–120 μm), and the ice cream mix with the addition of hydrolysates obtained using acid and lactase (8–10, 20–40, 60–70, and 80–90 μm). While for the ice cream mixes with the addition of ι-carrageenan and ι-carrageenan after β-galactosidase treatment, three peaks were noted (particle size ranges for I sample: 2–5, 15–30 and 90–110 μm and for L sample: 2–5, 7–15 and 80-110 μm. Moreover, the range and location of peaks were similar for samples C, I, and B. The samples A and L, with the hydrolysates obtained using acid and lactase, respectively, had also similarities in particle size distribution. After maturation, four peaks in particle size distribution were noticed only for the control sample, whereas three-peaks were detected for the other samples (Figure 2). Among the samples with stabilisers, the significant differences in particle size distribution were only visible for the samples with the addition of ι-carrageenan after acid treatment (A) and after lactase treatment (L). In both mixes, particles in the size ranges 10–40, 50–70 and 80–90 μm were dominant. The frequency of particles with diameter less than 5 μm was low. The more frequent particles between 10 to 100 μm could represent fat agglomerates or fat coalescence. The same observation was made for molten ice creams; single fat droplets with a size of 1–1.5 μm and fat clusters with a diameter of around 10 μm were determined [Liu et al., 2022]. Mendez-Velasco & Goff [2012] provided a deep analysis of the influence of the fat structure and properties of ice cream. Unsaturated monoglycerides (from sunflower oil) and saturated monoglycerides (from palm kernel oil) added to ice cream were tested. Based on particle size distribution, it was concluded that ice cream with unsaturated lipids formed larger fat networks with the higher number of droplets as stable particles, while ice cream with saturated lipids destabilized readily with smaller aggregates. The particles with diameters of 0.01–1 and around 100 μm dominated in ice cream with unsaturated lipids, while distribution of particle size was more homogenous in the ice cream with saturated lipids. In our study, the mix of saturated and unsaturated acids (mono- and diglycerides of fatty acids) was used as an emulsifier, which may explain such a wide range of particle sizes in all samples.
The rheological properties of ice cream mixes
The flow behavior and mouthfeel of ice cream are referred to as rheological properties. The rheological behaviour of milk ice cream mixes was described using the Ostwald de Waele model. The accuracy of this model fitting was high, as indicated by high correlation coefficients (Table 2). The rheological parameters of ice cream mixes before and after maturation are shown in Table 2. Data obtained for the samples before maturation shows that consistency index (K) ranged from 0.009 to 0.083. The addition of stabilisers contributed to an increase in its values owing to the fact that the lowest value was noted for the control sample. Considering the type of stabilizer used, the highest K was determined for the ice cream mix with the addition of ι-carrageenan after lactase treatment (L). In the case of the flow behaviour index of the samples before maturation, the lowest value was determined for L (0.727), but the values achieved for ice cream mix with ι-carrageenan and its acid hydrolysate were similar and did not differ significantly (p≥0.05) from that determined for L sample. The highest (p<0.05) n was determined for the control sample (0.900), which allows concluding that higher K was obtained from the lower flow behaviour index. In the research by Atalar et al. [2021], flow curves plotted for the hazelnut vegan ice cream mixes were described using the Ostwald de Waele model, and the values of their flow behaviour index ranged from 0.61 to 0.76. Similar n values (0.65–0.73) were computed for vanilla ice creams with various contents of milk protein concentrate [Mostafavi et al., 2017].
After maturation, the same trend in the variation of K and n between samples as before maturation was observed. The highest K value (0.104) was determined for the ice cream mix with the addition of ι-carrageenan after lactase treatment and the lowest one (0.012) for the control sample. In the case of the flow behaviour index, the lowest value (0.702) was noted for the L sample and the highest one (0.838) for the control sample. Soukoulis et al. [2008] showed that the consistency index of the ice cream mixes with the addition of different hydrocolloids and κ-carrageenan ranged from 0.498 to 1.951, which was due to the distinctive ability of κ-carrageenan to form a gel with other hydrocolloids. Thus, the combination of carrageenan with guar gum or carboxymethyl-cellulose should be more favourable than the combination with xanthan gum or sodium alginate, which usually forms weak gel networks. Referring to the views of Alvarez et al. [2005], after maturation, an increase in viscosity was observed in milk ice cream mixes. Such a change was understandable due to the hydration of protein and stabilisers during maturation, which results in a change in viscosity.
According to the presented results for all ice cream mixes (before and after maturation), the n values were less than 1 (Table 2). It means that the ice cream mixes showed non-Newtonian shear-thinning (pseudoplastic) behaviour [Rao, 2007]. Previous research reported that ice cream mixes exhibited pseudoplastic behaviour due to the aggregated fat globules and polysaccharide stabilisers [Akbari et al., 2019]. Consequently, the viscosity decreased with an increasing shear rate.
After maturation, only the samples with the addition of enzymatic hydrolysates of ι-carrageenan (B and L) tended to increase their consistency index (Table 2). It may suggest the same mechanism of rheological behaviour in both ice cream mixes. Our previous study [Kot et al., 2022] proved that the mentioned enzymatic hydrolysates of ι-carrageenan were more flexible in comparison to ι-carrageenan or acid hydrolysate of ι-carrageenan in model solutions of ice cream. It may be the reason why the consistency of samples B and L was improved.
Microscopic analysis
The microscopic observation of emulsion may provide pivotal clues to understanding the relationship between microstructure and the stability of the emulsion [Ahn et al., 2022]. The images from the CLSM analysis of the milk ice cream mix before and after maturation are presented in Figure 2. To observe the changes in the stability of samples, Nile red was used to stain the fat droplets. In all samples both before and after maturation, single particles of fat were visible with a size of around 5 μm. This is in agreement with the particle size distribution; the first peak was observed for the particles <10 μm (Figure 3) but larger fat agglomerates were visible in microscopic images as well (Figure 3). Their presence in ice cream mixes was also proven by previous analyses of the TSI and D50. The sizes of agglomerates were around 10 μm or more. Such molecules were visible in the control sample and ice cream mix with the addition of ι-carrageenan before maturation (Figure 3 Ca and Ia, respectively). Additionally, fat droplet deformation was observed in the image of the control sample before maturation. This confirmed flocculation, which was suggested above based on the backscattering profile. Moreover, as can be seen from image Ba, phase separation in the sample with ι-carrageenan after β-galactosidase treatment (before maturation) occurred, which also confirmed the destabilisation of this milk ice cream mix. Frising et al. [2008] noticed in the picture of sedimentation of water-in-oil emulsion that droplets which were not evenly distributed tended to gather in more or fewer agglomerates. It means that flocculation may occur in this place later. In our study, such phenomena could also be observed during maturation (Figure 2). Melted ice cream was also observed by confocal scanning laser microscopy after dynamic freezing [Voronin et al., 2020]. Based on the results, the control sample and the sample with the addition of polysorbate 80 (C-P80) were characterized by destabilized fat globules and partial coalescence, especially in the sample with the addition of an emulsifier. The same method was used to observe the protein and fat globules in ice cream mixes with polysaccharides [Cheng et al., 2015]. According to the results, flocculation was visible in the examined samples. In our research, the same or similar behaviour of fat destabilisation was observed.
CONCLUSIONS
The presented study showed that ι-carrageenan and its hydrolysates had a strong influence on the physical properties of milk ice cream mixes. They affected the stability of the emulsion during maturation. The hydrolysates of ι-carrageenan improved the stability of mixes contrary to ι-carrageenan as indicated by TSI. On the other hand, the addition of ι-carrageenan to the mixes affected two different types of destabilisation: coalescence and flocculation, while in the samples with hydrolysates of ι-carrageenan additionally, sedimentation was noted. Before and after maturation, aggregates of fats occurred and particles of distinctive sizes were observed in the ice cream mixes. The used stabilisers contributed to increasing the sizes of particles while maturation decreased it. The addition of the enzymatic hydrolysate of ι-carrageenan after commercial lactase treatment beneficially influenced the rheological properties of the ice cream mix.
It may be concluded that ι-carrageenan and its hydrolysates could serve as effective stabilisers in milk ice cream. However, further investigations are needed to explain the mechanism of their action as stabilisers and the behavior of fat in the presented recipe during maturation. The study ultimately proved that the type of stabilisers, in this case, ι-carrageenan and its hydrolysates, contributed to the destabilisation of ice cream mixes during maturation. Such a conclusion may be useful in planning the ice cream production process or predicting the formation of ice crystal structure.

