INTRODUCTION
Plants have been a component of human diet since the earliest times. Strong passion for consuming plant products is noticeable in Asian countries, especially in Japan, Korea, China, and Vietnam, where the phenomenon of herbophilia has been observed for years [Pinakin et al., 2020]. Also, a common phenomenon is the consumption of edible flowers, called florifagia. Edible flowers (EF) are defined as flowers that can be safely consumed by humans [Lu et al., 2016; Purohit et al., 2021]. However, despite the fairly widespread use of flowers as food, there is still no general classification of them into edible and inedible. Edible flowers are niche products in Poland, due to their low availability (seasonality) and no traditions of their consumption. They are still treated as a decorative element of dishes and not as a source of nutrients. Nevertheless, in Poland, as in the other European countries, consumer interest in EF and dishes based on them has increased in recent years. The market for the sale of EF is expanding and, accordingly, adequate packaging for transport and trade needs to be ensured. The EF are usually packed individually and in flower boxes [Fernandes et al., 2020b].
The interest in EF is growing mainly due to their aesthetic properties: unique flavours, aromas, and colours, as well as their nutritional values [Takahashi et al., 2020]. EF are a rich source of carbohydrates and proteins, and do not contain excessive lipids. They also contain dietary minerals (calcium, iron, potassium, magnesium, phosphorous, and zinc) and vitamins [dos Santos et al., 2018; Purohit et al., 2021]. Moreover, EF represent a source of many biologically active substances, including phenolic compounds [Fernandes et al., 2017; Lu et al., 2016; Mlcek & Rop, 2011]. Therefore, there are numerous health benefits from EF consumption, due to their antianxiety, anticancer, antidiabetic, anti-inflammatory, antioxidant, diuretic, anthelmintic, immunomodulatory, and antimicrobial effects [Demasi et al., 2021a; Devecchi et al., 2021; Lu et al., 2016; Navarro-González et al., 2015; Pinakin et al., 2020; Skrajda-Brdak et al., 2020; Zhao et al., 2021].
It should be remembered, however, that in addition to compounds with a health-promoting effect, EF can be a source of anti-nutritional and even toxic substances [Klintschar et al., 1999; Kristanc & Kreft, 2016; Lara-Cortés et al., 2013; Sotelo et al., 2007]. They may contain environmental pollution residues (such as heavy metals), as well as substances used in agriculture (pesticides, fertilizers) and various types of pathogenic microorganisms [Matyjaszczyk & Śmiechowska, 2019]. Due to the high content of water and nutrients, EF provide an excellent medium for many species of microorganisms, including yeasts and moulds. The EF processing may increase the risk of contamination with, among others, contaminants, total aerobic mesophilic bacteria, Salmonella sp., Escherichia coli, coliforms, yeasts, and moulds [Fernandes et al., 2020a]. Subsequently, the use of contaminated EF, as an ingredient in ready-to-eat food, contributes to food spoilage and may cause foodborne outbreaks [Matyjaszczyk & Śmiechowska, 2019; Wilczyńska et al., 2021]. At this point, it should be emphasized that there are no requirements for the production process and conditions for harvesting and storing edible flowers. Legal regulations relating to quality and safety assurance for EF consumption have not been developed; only Regulation (EC) No. 258/97 of the European Parliament and of the Council of 27 January 1997 concerning novel foods and novel food ingredients, provides some information about the safety of EF [Regulation (EC), 1997].
EF are usually consumed either fresh or minimally processed [Fernandes et al., 2020b; Takahashi et al., 2020]. Low-temperature storage is widely used as an effective preservation method to maintain the quality and extend the shelf life of EF [Fernandes et al., 2019; Shantamma et al., 2021]. The packaging of EF, as in the case of other fresh or minimally processed products, plays also an important role in maintaining their quality – it is a barrier between the product and the external environment and protects them against pollution, mechanical damage, and desiccation [Hussein et al., 2015]. The type of packaging materials is an important factor in controlling flower deterioration. Therefore, the aim of this study was to evaluate the effect of the vacuum-sealed polyamide/polyethylene (PA/PE) bag and polyethylene terephthalate (PET) box packaging on the microbial contamination of selected edible flowers during refrigerated storage.
MATERIALS AND METHODS
Collection and storage of flowers
The research materials consisted of selected edible flowers: nasturtium (Tropaeolum L.), calendula (Calendula officialis L.), and daisy (Bellis perennis L.), which were freshly harvested during the 2021 harvest season directly from a commercial farm – Ogrodnictwo Lawenda, Gdańsk, Poland. The flowers were picked by hand while maintaining hygiene rules. Immediately after collection, all samples were packed in disposable food packaging (PET boxes) and then transported in a portable refrigerator to the laboratory, where the flowers were divided into three groups. One group was used for microbiological tests (day 0); from each type of flowers, 10 g were taken twice in order to obtain parallel replicates (n=6). The second group was packed in PET boxes (3 boxes of each type of flowers, 50 g each), and the third was packed in vacuum-sealed PA/PE bags (3 bags of each type of flowers, 50 g each) using a CVP-350/MS vacuum packer (CAS, Warsaw, Poland). The packed flowers, both in PET boxes and vacuum-sealed PA/PE bags, were kept in a refrigerator at 4±0.5°C for 3 days. On each day of storage, two parallel samples of 10 g of each type of flowers were taken from both PA/PE bags and PET boxes for microbiological tests (n=36). The whole experiment was repeated three times, at weekly intervals, producing a total of 126 samples that were analysed. The appearance of the stored flowers (colour, freshness, and wilting) was assessed through a daily visual inspection made by the same person.
Microbiological analyses
Microbiological analyses were performed on day 0 (control) and 1, 2, and 3 days of flower storage according to the methodology detailed in our previous article [Wilczyńska et al., 2021]. Ten grams of each flower type were collected in a laminar air flow chamber and then homogenised together with 90 mL of a Ringer’s solution using a Stomacher lab-blender 400 (Seward, Worthing, United Kingdom). Homogenates were subjected to further dilution. The counts of Staphylococcus aureus, Escherichia coli, yeasts, and moulds on the flowers was determined using the pour-plate technique. The conditions for carrying out analyses are presented in Table 1. The presence of Salmonella sp. was determined using chromogenic Brilliance™ Salmonella agar for isolation and initial differentiation. Salmonella sp. were pre-multiplied in buffered peptone water for approx. 24 h at 35°C, and then 0.1 mL was placed on the prepared chromogenic medium and incubated at 35°C for 48 h. The results of these analyses were given as the logarithm of colony-forming units per gram of flower (log cfu/g).
Table 1
The conditions of microbiological analyses conducted with the pour-plate technique.
Statistical analysis
The mean and standard deviations were calculated using Statistica v. 13.1 software (StatSoft, Tulsa, OK, USA). One-way and two-way analysis of variance (ANOVA) was conducted to investigate the overall effect of packaging type and storage time (days) on the extent of EF contamination with individual microorganisms. The Tukey test was used to determine significant differences between means (p˂0.05).
RESULTS AND DISCUSSION
The visual assessment of the flowers showed that slight changes took place during the three-day refrigerated storage of nasturtium and calendula. Flowers packed both in PET boxes and vacuum-sealed PA/PE bags gently turned brown and withered. Only the daisies were unchanged in colour and showed faint signs of wilting (Figure 1). However, further sensory analysis should be carried out by qualified panellists to check how the appearance and condition of flowers will be affected by prolonged storage under refrigerated conditions. According to Demasi et al. [2021b], the visual appearance of most of the EF (thirteen species out of the 17 analysed in their study, including nasturtium and calendula) should be acceptable for seven days if stored at 4°C.
Figure 1
Flower appearance after 3 days of refrigerated storage; A – nasturtiums in vacuum-sealed polyamide/polyethylene (PA/PE) bags, B – calendula in vacuum-sealed PA/PE bags, C – daisy in vacuum-sealed PA/PE bags, D – nasturtiums in polyethylene terephthalate (PET) box, E – calendula in PET box, F – daisy in PET box.

The degree of microbiological contamination of the EF on the harvesting day is shown in Table 2. None of the flowers was contaminated with Salmonella sp., which proves that proper agricultural and hygienic practices were followed in EF production and harvesting. According to Matyjaszczyk & Śmiechowska [2019], all cases of microbial contamination of EF recorded in the Rapid Alert System for Food and Feed (RASFF) were associated with the presence of different Salmonella sp. serotypes. Salmonella sp. is a bacterium that is quite common in the environment, but the sources of infection are mainly food products of animal origin [Ehuwa et al., 2021]. It is an indicator of the hygienic condition of food products. In the case of products of plant origin, contamination with this bacterium may occur during cultivation and various post-harvest operations. The sources of contamination with Salmonella during processing and post-harvest operations may include workers, equipment as well as inadequate process management and packaging [Ehuwa et al., 2021; Popa & Popa, 2021]. A major contributor to foodborne illness outbreaks is post-processing contamination [Jung et al., 2014; Matyjaszczyk & Śmiechowska, 2019].
Table 2
Counts of Escherichia coli, Salmonella sp., Staphylococcus aureus, yeasts, and moulds on flowers (log cfu/g) on harvest day.
| Flower | Escherichia coli | Salmonella | Staphylococcus aureus | Moulds | Yeasts |
|---|---|---|---|---|---|
| Nasturtium | 1.19±1.48 | nd | 2.05±0.56b | 5.06±0.48a | 4.48±0.76a |
| Calendula | nd | nd | 1.89±0.16b | 4.40±1.07a | 4.18±1.12a |
| Daisy | nd | nd | 2.72± 0.32a | 3.90±0.26b | 4.56±0.59a |
The results indicate that the nasturtium was the only flower type contaminated with E. coli (Table 2). Fernandes et al. [2020a] reported that counts for total coliforms and E. coli were less than 1 log cfu/g in the fresh flowers they analysed. E. coli is one of the most common indicators of food contamination, the absence of E. coli proves that the sanitary conditions or hygiene practices during or after food production have been maintained. The presence of coliforms on nasturtiums in our study, may be due to the fact that they were grown in a site richly fertilized with natural manure, which could be a source of contamination.
The count of S. aureus ranged from 1.89 log cfu/g in calendula flowers to 2.72 log cfu/g in daisies (Table 2) and was significantly higher in daisies (F=328.53, p=0.00004). These contamination rates of EF with S. aureus were smaller than contamination rates of ready-to-eat salads estimated at an average of 3 log cfu/g by Habibi Najafi & Bahreini [2012] and Saifullah et al. [2018]. The presence of S. aureus is an indicator of poor hygienic conditions, as this bacterium is mainly transferred by personnel in contact with food [Habibi Najafi & Bahreini, 2012]. According to Microbiological Guidelines for Food [2014], the acceptable contamination level with S. aureus in ready-to-eat foods should not exceed 20 cfu/g, while the level observed in EF (approx. 2 log cfu/g) in the present study is defined as borderline. All samples were contaminated with S. aureus, which could lead to foodborne infection.
All flowers were contaminated with moulds at counts ranging from 3.90 to 5.06 log cfu/g, and with yeasts at counts ranging from 4.18 to 4.56 log cfu/g (Table 2). Mould number was significantly lower in daisies (F=3.76, p=0.03), while the number of yeasts did not differ significantly between the flower types (p≥0.05). Habibi Najafi & Bahreini [2012] who tested ready-to-eat salads and fresh herbs in Mashhad (Iran), isolated a similar amount of yeasts and moulds at levels ranging between 3.85 and 6.7 log cfu/g. Adeyemi et al. [2019] reported that the fungal count ranged from 4.22 to 4.46 log cfu/g in fresh salads. In turn, Kowalska & Szczech [2022] detected moulds in 106 (87%) samples of leafy green vegetables and found the highest average count of moulds (5.40 log cfu/g) in rocket. In the last mentioned publication, it was also reported that almost all the vegetables examined were contaminated with yeasts (with the exception of two iceberg lettuce samples and one chive sample), and the average yeast count was the highest in lamb’s lettuce, parsley, and rocket – 5.94, 5.63, and 5.62 log cfu/g, respectively. Lara-Cortés et al. [2016] determined the fungal count of about 5.7 log cfu/g on dahlia packaged in PET. In the present study, a similar number of fungi was detected in nasturtium while counts of yeasts and moulds in daisies and calendula were lower by around 1.5 logarithmic unit. Fernandes et al. [2020a] reported average counts of yeasts and moulds in fresh edible flowers at the level of 2 log cfu/g, which was much lower than in the present study. Additionally, it was found that the low-temperature conditions (–18°C) had a protective effect against the growth of some spoilage microorganisms and foodborne pathogens, as their counts decreased or remained the same after three-month storage under these conditions for most of the flowers.
Comparing the present study results with the general guidelines for the number of non-pathogenic microorganisms in various types of unprocessed vegetables and fruits causing spoilage of these products (established for moulds and yeasts at ≤103 cfu/g) [Ragaert et al., 2010], it can be concluded that EF are heavily contaminated with these microorganisms. EF can be contaminated with moulds and yeasts at any stage of their life cycle: during cultivation, harvest, transport, and marketing [Tournas, 2005]. High levels of fungi found on flowers may affect their nutritional and sensory characteristics. This is because these microorganisms change and accelerate the aging process of stored flowers and can produce volatile compounds responsible for off-flavours and texture changes [Rawat, 2015]. The presence of moulds and yeasts is also undesirable due to their potential adverse effects on human health – some of them (Aspergillus, Penicillium, Alternaria or Fusarium) may produce mycotoxins and others may cause allergies (Cladosporium, Alternaria) [Habibi Najafi & Bahreini, 2012; Kowalska & Szczech, 2022; Tournas, 2005].
The types of flowers differed in terms of the count of moulds and S. aureus. It is difficult to identify the causes of these differences. In the world literature, there are only few articles on the microbial contamination of edible flowers. Some species of flowers may be resistant to microbiological inoculation due to the content of phenolic compounds. It has been proven that flavonoids, such as flavanones, flavones and flavonols, inhibit the growth of Candida albicans and Aspergillus flavus [Cushnie & Lamb, 2005; Prasad et al., 2014; Selma et al., 2009]. Quercetin inhibits the development of pathogenic microorganisms, e.g. S. aureus, Enterococcus faecalis, Bacillus subtilis, E. coli. Anthocyanins, in turn, exert bacteriostatic and bactericidal effects against many microorganisms (including Staphylococcus, Klebsiella, Helicobacter, Bacillus). Phenolic compounds also elicit an inhibitory effect on viruses [Cushnie & Lamb, 2005]. The contents of the above-mentioned compounds in the tested flowers vary from low to high; therefore, they may have different inhibitory effects against microorganisms [Pires et al., 2021].
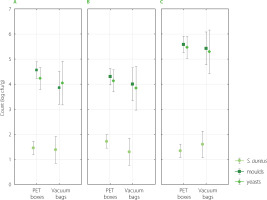
The counts of S. aureus, yeasts, and moulds on edible flowers on harvesting day and during successive storage at low temperature (4°C) are shown in Figure 2. Flowers stored for 3 days had similar counts of S. aureus, yeasts, and moulds. A slight decrease in the number of moulds and yeasts was noticed only in the case of nasturtium flowers (by 1 log cfu/g), but it was not a significant (p≥0.05) decrease. Figure 3 shows the differences in counts of S. aureus, yeasts, and moulds on edible flowers packed in different packagings (PET box and vacuum-sealed PA/PE bags) after 3-day storage. The type of packaging did not significantly (p≥0.05) affect the number of microorganisms either. The counts of S. aureus, moulds, and yeasts were slightly lower after three days of refrigerated storage in vacuum-sealed PA/PE bags, but these differences were not statistically significant (p≥0.05). The two-way analysis of variance also showed that the combination of these factors (storage time and packaging type) had no significant effect on the development of microflora (p≥0.05).
Figure 2
Counts of Staphylococcus aureus, yeasts, and moulds on edible flowers on harvesting day and after 1–3-day refrigerated storage (4°C); A – nasturtium, B – calendula, C – daisy.
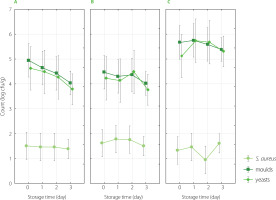
Figure 3
Counts of Staphylococcus aureus, yeasts, and moulds on edible flowers packed in polyethylene terephthalate (PET) boxes and vacuum-sealed polyamide/ polyethylene (PA/PE) bags after 3-day refrigerated storage (4°C); A – nasturtium, B – calendula, C – daisy.
Packaging is the main tool to protect flowers from desiccation and to preserve their frail structure. It provides a barrier between flowers and the outside environment, thereby minimising their exposure to pathogens and other contaminants [Hussein et al., 2015]. Microbes have different growth requirements; hence, the use of different packaging methods can delay or prevent their growth [Fernandes et al., 2019]. However, bacteria and fungi are unpredictable and some of them can still grow despite the measures taken, and cause spoilage of edible flowers [Rawat, 2015]. Also low-temperature storage is one of the easiest and simplest methods of EF preservation. It was observed that EF could be satisfactorily stored for 7 to 14 days at 4°C depending on species [Demasi et al., 2021b]. The present study confirmed that combining two methods of preservation – storage in proper conditions (time, temperature) and packaging allowed maintaining an unchanged level of microbial contamination: there were no significant changes in the number of bacteria and moulds and yeasts after 3 days of refrigerated storage. However, there still remains the problem of preserving the appearance of the flowers during storage – how to store EF so that they retain their freshness and proper sensory characteristics as long as possible.
CONCLUSIONS
The present research indicated that the most important factors influencing the microbiological population on edible flowers, and thus the safety of their consumption, are pre-harvest factors. However, proper packaging and refrigerated storage for 3 days kept the microbiological contamination level unchanged. Neither the type of packaging nor storage in refrigerated conditions influenced the extent of microbial contamination of flowers. However, further research should be carried out into the long-term storage of packaged edible flowers under refrigeration conditions. It is also necessary to look for other ways to preserve EF. Taking into account results shown, it is necessary to inform the flowers suppliers that the level of microbiological contamination of flowers depends primarily on the growing conditions, but proper post-harvest treatment (appropriate harvesting, packaging, and storage) prevents cross-contamination and inhibits the further development of microorganisms.

