INTRODUCTION
The use of plants with bioactive properties has a rich historical backdrop and continues to play a crucial role in contemporary health practices. Among such plants, Piper sarmentosum, commonly known as betel leaf, stands out for its diverse therapeutic benefits. Native to Southeast Asia, P. sarmentosum has been used in traditional treatments for its anti-inflammatory, antimicrobial, and antioxidant properties [Sun et al., 2020]. Phytochemical analysis of Piper sarmentosum reveals the presence of various bioactive compounds, including flavonoids and phenolic acids, which contribute to its health-promoting effects [Adib et al., 2024]. These compounds are known to exhibit a range of beneficial activities, including anti-cancer, anti-diabetic, and liver-protective effects [Adib et al., 2024; Othman et al., 2022].
Kombucha, a fermented tea beverage, has gained global popularity for its potential health benefits [Kapp & Sumner, 2019]. Originating from Asia, kombucha is produced through the fermentation of a sweetened tea by a symbiotic culture of bacteria and yeast (SCOBY). The fermentation process results in the formation of organic acids such as acetic, gluconic, and lactic acids, as well as bioactive compounds like phenolic acids and flavonoids, which are credited with the drink’s health-promoting properties [Coelho et al., 2020]. These include antioxidant and antimicrobial effects, which contribute to its potential in supporting gut health and enhancing overall immunity [Coelho et al., 2020; Kitwetcharoen et al., 2023].
While traditional kombucha is typically made using tea from Camellia sinensis, recent innovations have explored the use of alternative substrates to create diverse kombucha varieties [Bortolomedi et al., 2022]. Herbs, fruits, and vegetables have been employed to enrich the nutritional profile and therapeutic potential of kombucha [Emiljanowicz & Malinowska-Pańczyk, 2020]. P. sarmentosum, with its rich profile of beneficial phytochemicals, presents a promising candidate for this purpose [Sun et al., 2020]. Incorporating P. sarmentosum into kombucha could potentially offer a functional beverage that combines the probiotic benefits of kombucha with the bioactive properties of P. sarmentosum.
This study aimed to develop and evaluate a novel kombucha variant using P. sarmentosum as a primary ingredient. The phytochemical composition, bioactivity including antioxidant, antibacterial, and anti-amylase activities, and sensory acceptability of P. sarmentosum-based kombucha (PSK) were investigated. By exploring this innovative approach, we sought to enhance the functional food and beverage market with a product that offers synergistic health benefits, thus contributing to the advancement of nutritionally enriched beverages.
MATERIALS AND METHODS
Preparation of Piper sarmentosum-based kombucha
P. sarmentosum was grown and harvested from experimental garden of the Department of Biotechnology, NTT Hi-tech Institute, Nguyen Tat Thanh University, Ho Chi Minh City, Viet Nam. The harvested leaves were dried at 40°C in a hot air oven until the moisture content was reduced to below 10 g/100 g, ensuring suitability for kombucha preparation. The starter cultures, provided by Foodplus Ltd., Hanoi, Vietnam, included a sour broth and a cellulosic pellicle stored at 4°C. This SCOBY comprises various microorganisms, including the yeast species: Brettanomyces bruxellensis and Saccharomyces cerevisiae, as well as the acetic acid bacteria (AAB): Komagataeibacter pomaceri and Komagataeibacter rhaeticus. The microbial content was verified at approximately 5×106 CFU/g by the Institute of Microbiology and Biotechnology, Vietnam National University, Hanoi (VNU), Vietnam.
Dry P. sarmentosum leaves were used to prepare the tea infusion. To prepare this infusion, 10 g of the leaves were steeped in 1,000 mL of hot water for 20 min. The infusion was then filtered using a sterile sieve. Sucrose was added at a concentration of 100 g/L and fully dissolved in the tea infusion. After cooling to ambient temperature, the mixture was divided into small bottles (90 mL each). To each bottle, 3 g of SCOBY (3%) and 10 mL of sour broth (10%) were added. Kombucha beverages were fermented at 30°C and observed at 0, 7, 14, and 21 days to represent the commonly studied stages of fermentation [Valiyan et al., 2021]. Three independent batches were prepared and analyzed.
Fermentation monitoring by measuring SCOBY growth, sugar content, pH, and total acidity
During the fermentation period (days 0, 7, 14, and 21), samples of the cellulosic pellicle were collected from each batch to measure SCOBY production (g/L). The sugar content (°Brix) of each sample was measured using an RA500 KEM refractometer (Kyoto Electronics Manufacturing Co.,Ltd., Kyoto, Japan) at 20°C. The pH values of the kombucha beverages were measured with a Mettler Toledo J12683 pH meter (Mettler-Toledo International, Inc., Greifensee, Switzerland). Total acidity was assessed through potentiometric titration and expressed as g of acetic acid equivalents per 100 mL. This was achieved by titrating with 0.1 M NaOH until the pH reached 8.1. All analyses were performed in triplicate.
Determination of total phenolic and total flavonoid contents
The total phenolic content (TPC) in PSK was measured using the colorimetric assay with the Folin–Ciocalteu reagent [Singleton & Rossi, 1965]. In this method, 100 µL of the fermentation samples were combined with 900 µL of distilled water and 500 µL of 10% Folin–Ciocalteu reagent. Then, 500 µL of a 7.5% Na2CO3 solution was added. The final mixture was incubated at 40°C for 30 min. The absorbance of the reaction mixture was measured at a wavelength of 765 nm by a C-7000UV spectrometer (Peak Instruments Inc.,Houston, TX, USA). The TPC was quantified using a gallic acid standard curve, with results expressed as mg of gallic acid equivalents (GAE) per L of kombucha (mg GAE/L).
The total flavonoid content (TFC) was quantified using a colorimetric method with AlCl3 [Amjadi et al., 2023]. For this assay, 0.5 mL of each kombucha beverage was diluted in 1.5 mL of 99% ethanol and allowed to stand for 5 min. Next, 0.1 mL of 10% AlCl3 was added, and the mixture was left at room temperature for 5 min. Subsequently, 0.1 mL of 1 M CH3COOK and 2.8 mL of distilled water were introduced to the solution and incubated for 45 min at ambient temperature. The absorbance of the final reaction mixture was measured at 415 nm by a C-7000UV spectrometer (Peak Instruments Inc.). A quercetin standard solution was used to construct the standard curve for TFC quantification, with results expressed as mg of quercetin equivalents (QE) per L of kombucha (mg QE/L).
Assessment of antioxidant activity
The assessment of free radical scavenging activity in PSK was conducted using spectrophotometric methods, specifically the 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay and the 2,2’-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) assay.
The DPPH assay was conducted following the method developed by Brand-Williams et al. [1995] with slight modifications. The portion of 50 µL of each diluted PSK or Trolox solution was mixed with 1 mL of a DPPH radical solution (40 mg/L, TCI Chemical Co., Japan). After incubating the mixture in a dark environment at ambient temperature for 30 min, its absorbance was measured at a wavelength of 517 nm by a C-7000UV spectrometer (Peak Instruments Inc.).
The ABTS assay followed the methodology outlined by Re et al. [1999]. The ABTS (Cool Chemical Science and Technology,Beijing, China) solution at a concentration of 7 mM was mixed with a 2.45 mM K2S2O8 solution and stored in darkness for 16 h before use. For the assay, 1 mL of the prepared ABTS radical cation solution was combined with diluted PSK or Trolox. After incubating the mixture in the dark at room temperature for 6 min, its absorbance was measured at 734 nm by a C-7000UV spectrometer (Peak Instruments Inc.).
The results of both assays were expressed as µmol of Trolox equivalent (TE) per mL of kombucha.
Evaluation of antibacterial efficacy
The antibacterial efficacy of PSK was assessed using the agar diffusion method against several pathogenic bacterial strains: Escherichia coli, Salmonella typhi, Vibrio cholerae, and Staphylococcus aureus. These bacteria were first cultured overnight in Mueller Hinton Broth (HiMedia Laboratories, Mumbai, India). The bacterial cultures were then suspended in phosphate-buffered saline (PBS) and diluted to achieve a concentration of 1×107 CFU/mL.
For the antibacterial assay, 100 μL of each bacterial suspension were evenly spread on Mueller Hinton Agar (MHA) plates. Sterile metallic tubes were used to create three wells, each 5 mm in diameter, on the agar plates. Following this, 100 μL of each P. sarmentosum tea kombucha sample were added to the wells to enable diffusion into the agar. Sterile distilled water served as the control. The plates were then incubated at 37°C for 24 h. The antibacterial activity was determined by measuring the zones of inhibition around each well.
Assessment of α-amylase inhibition activity
The inhibition of α-amylase activity was assessed via the starch-iodine test, following the methodology described in a previous study [Do et al., 2024]. In this assay, 400 µL of the PSK were mixed with 400 µL of a 4 μM α-amylase solution (Sigma-Aldrich, St Louis, MO, USA) in a 0.04 M phosphate buffer solution at pH 6.9. This mixture was incubated at 36°C for 10 min. Subsequently, 400 µL of a 1% starch solution was added, and the mixture was incubated again at 36°C for another 10 min. The reaction was halted by adding 100 µL of 1 M HCl. To develop the colour, 100 µL of a 5 mM iodine solution was added to 200 µL of the reaction mixture, followed by dilution with 1 mL of distilled water. The absorbance was measured at 570 nm using a C-7000UV spectrometer (Peak Instruments Inc.). Phosphate-buffered saline (PBS) served as the negative control, while acarbose (1 mg/mL) was used as the positive control. The inhibition of α-amylase activity was calculated using Equation (1):
where: Ac is the absorbance of the negative control (enzyme + substrate, no inhibitor), Acb is the absorbance of the control blank (substrate without enzyme or inhibitor), As is the absorbance of the test sample (enzyme + substrate + inhibitor), and Asb is the absorbance of the sample blank (substrate + inhibitor, no enzyme).Sensory evaluation
The sensory properties of PSK were evaluated by a panel of 20 trained assessors (10 men and 10 women, aged 22–35 years), selected from graduate students and faculty members of the Department of Food Technology, Institute of Applied Technology and Sustainable Development, Nguyen Tat Thanh University, Ho Chi Minh City. The panelists had prior experience with sensory evaluation and underwent an additional training session to familiarize themselves with the evaluation process and the specific attributes of the kombucha. During the training, the meaning and evaluation criteria for each sensory attribute (appearance, color, odor, sweetness, sourness, astringency, and overall sensory acceptability) were explained using reference samples to standardize interpretations and avoid misjudgments. A 9-point hedonic scale, ranging from 1 (dislike extremely) to 9 (like extremely), was used to rate the intensity of each characteristic [Panda et al., 2017].
The assessments were conducted in a sensory evaluation laboratory designed to minimize external distractions, equipped with uniform lighting and air circulation. The kombucha samples, representing three batches of PSK for 7, 14, and 21 days, were coded with three-digit random numbers to ensure unbiased evaluation. Samples were served in clean, transparent tumblers. Between assessments, panelists were provided with water and unsalted crackers to cleanse their palates. Panelists were instructed not to discuss their scores during the assessment sessions.
Statistical analysis
The data obtained from the analysis were processed and reported as the means and standard deviation (SD)from three experimental replicates (representing three different fermentation batches of PSK, fermented for 7, 14, and 21 days). Statistical significance between different fermentation times was determined using one-way analysis of variance (ANOVA) followed by Tukey’s test with a significance threshold set at p≤0.05. All statistical analyses and graph generation were performed using GraphPad Prism 8 (GraphPad Software, La Jolla, CA, USA).
RESULTS AND DISCUSSION
Changes in SCOBY mass, pH, acetic acid content, and sugar content during fermentation
During the fermentation of the sweetened infusion of P. sarmentosum leaves,the indicators including SCOBY mass, pH, total acidity, and sugar content were monitored and recorded at days 0, 7, 14, and 21 (Figure 1). The weight of the SCOBY layer showed a significant (p≤0.05) increase beginning from day 7 and continuing throughout the 14 days of fermentation. The wet SCOBY weight increased from 27.3 g/L on day 7 to 95.3 g/L by day 14, remaining steady at 95.2 g/L on day 21. A similar trend was observed for the dry SCOBY. This accumulation of microbial biomass indicates that sweetened infusion of P. sarmentosum was an effective substrate for SCOBY formation. Previous studies demonstrated that the co-fermentation of whole-plant cassava with increasing levels of P. sarmentosum fostered a diverse microbial community increasing lactic acid bacteria count and shaping the predicted functional roles of the bacteria [Li et al., 2024]. Concurrently,fermentation leads to a rapid decrease in reducing sugar content in kombucha, as observed in our studies where sugar levels content diminished significantly (p≤0.05) over time (Figure 1B). This substantial reduction in sugar content suggests efficient microbial activity in PSK, beneficial for consumers seeking lower sugar intake.
Figure 1
Mass of symbiotic culture of bacteria and yeast (SCOBY) (A), sugar content expressed by Brix scale (B), pH (C) and total acidity (D) during production of Piper sarmentosum-based kombucha. Data are presented as the means and standard deviations (n=3). Different letters above bars/points indicate significant differences over fermentation time (p≤0.05).

The initial pH of thesweetened P. sarmentosum leaf infusion was 6.33, which decreased markedly over the fermentation period (Figure 1C). At the end of the fermentation period (day 21), the pH of PSK was approximately 2.55. The pH values of the PSK were within the standard safe range for human consumption, which is between 2.5 and 4.2 [Nummer, 2013]. The increase in the total acidity (Figure 1D) corresponded with the observed decrease in pH, indicating effective fermentation and robust acid production in PSK, essential for the microbiological safety and stability of kombucha [Mo et al., 2008].
Total phenolic and total flavonoid contents of kombucha
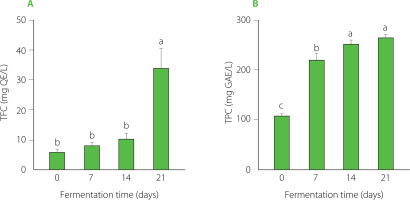
Phenolics are well-known for their antioxidant properties, which contribute significantly to the health benefits of kombucha [Kitwetcharoen et al., 2023]. Recent findings by Ware et al. [2024] revealed a rich profile of over 150 metabolites in P. sarmentosum leaves, predominantly consisting of flavonoids, which play a crucial role in the bioactive potential of the plant. During fermentation of the sweetened P. sarmentosum leaf infusion, the TFC started at approximately 6.02 mg QE/L on day 0, increasing to 7.96 mg QE/L and 10.28 mg QE/L on days 7 and 14, respectively, with no significant (p>0.05) changes observed during this period. The TFC then increased sharply, reaching a peak of 34.07 mg QE/L by day 21. Similarly, TPC in PSK also increased significantly throughout the fermentation period (Figure 2B). Initially determined at approximately 108.2 mg GAE/L on day 0, the TPC increased significantly to 221.4 mg GAE/L by day 7. It further increased notably to 251.27 mg GAE/L by day 14 and 266.2 mg GAE/L by day 21. Our data on PSK align with findings from kombucha made with black tea and alternative substrates like papaya leaves and wheatgrass, showing a significant increase in TFC and TPC during fermentation [de Noronha et al., 2022; Do et al., 2024; Sun et al., 2015]. Microbial enzymes-including glucosidase, pectinase, xylanase, cellulase, and glucanase, particularly from acetic acid bacteria and yeasts, hydrolyze the oligomeric and polymeric polyphenols and glycoside forms of phenolics in tea to simple phenolics and aglycons, such as catechin and epicatechin, thereby increasing the total phenolic and flavonoid contents [Chu & Chen, 2006; de Noronha et al., 2022; Kim et al., 2023]. While the increases in total phenolic and flavonoid content were notable, comprehensive metabolomic studies are crucial for the accurate identification and quantification of these bioactive compounds.
Figure 2
Total flavonoid content (TFC) (A) and total phenolic content (TPC) (B) in Piper sarmentosum-based kombucha at various fermentation times. Data are presented as the mean and standard deviation (n=3). Different letters indicate significant differences over fermentation time (p≤0.05). GAE, gallic acid equivalent; QE, quercetin equivalent.
Antioxidant activity
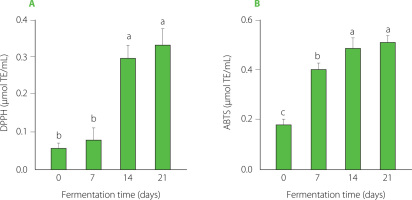
Antioxidants play a crucial role in scavenging free radicals that cause oxidative stress and damage to cells [Ifeanyi, 2018]. Kombucha is rich in such antioxidants, which are primarily phenolic compounds [Kitwetcharoen et al., 2023]. In our study, the DPPH assay results indicated an increase in antioxidant activity of the sweetened P. sarmentosum leaf infusion over the fermentation period (Figure 3A). At day 0, the antioxidant capacity was approximately 0.06 µmol TE/mL and increased significantly to 0.29 µmol TE/mL by day 14 and 0.33 µmol TE/mL by day 21. Similarly, the ABTS assay results show a significant (p≤0.05) increase in antioxidant activity throughout the fermentation period (Figure 3B). At day 0, the antiradical activity against ABTS cation radical was approximately 0.18 µmol TE/mL. It increased significantly to 0.40 µmol TE/mL by day 7, and further rose to 0.49 µmol TE/mL and 0.51 µmol TE/mL by days 14 and 21, respectively. This enhancement was likely a result of the increased content of phenolics including flavonoids during fermentation, as shown in Figure 2. A study by Huang et al. [2024] determined that the DPPH radical and ABTS radical cation scavenging activities of kombucha produced from black tea were the highest (2 and 3 µmol TE/mL, respectively) after 9 days of fermentation. In comparison, kombucha produced from P. sarmentosum exhibited lower DPPH radical and ABTS radical cation scavenging activates. This difference may primarily be due to the lower baseline antioxidant content in P. sarmentosum compared to C. sinensis. For example, prior to fermentation, the TPC of sweetened P. sarmentosum leafinfusion in our study was approximately 108 mg/L, while that of C. sinensis infusions, including green and black tea, was around five times higher [Hsieh et al., 2021]. While PSK had lower antioxidant activity than traditional kombucha, the substantial increase observed during fermentation highlights its potential as a functional beverage.
Figure 3
DPPH radical scavenging activity (A) and ABTS radical cation scavenging activity (B) of Piper sarmentosum-based kombucha at various fermentation times. Data are presented as the mean and standard deviation (n=3). Different letters denote significant differences over fermentation time (p≤0.05).
Antibacterial activity
Previous studies have shown that traditional kombucha from black tea [Battikh et al., 2013] and kombucha fermented from substrates of various herbs, such as thyme, fennel, rosemary, and mint, exhibited potential antimicrobial properties through the agar diffusion method [Battikh et al., 2012]. Extracts from P. sarmentosum leaves contain various secondary metabolites, such as glycosides, flavonoids, terpenoids, alkaloids, and phenolics, which have shown inhibitory effects against S. aureus [Yusof et al., 2019]. For the first time, we demonstrated the antimicrobial activity of kombucha derived from P. sarmentosum. We conducted tests against four common pathogenic bacteria: E. coli, S. typhi, V. cholerae, and S. aureus.
PSK showed no significant (p<0.05) antibacterial effect against E. coli and S. typhi throughout the fermentation period. The lack of effect on E. coli and S. typhi suggests that the antibacterial compounds produced during the fermentation of sweetened P. sarmentosum leaf infusion are not effective against these strains. Conversely, the antibacterial activity against S. aureus and V. cholerae exhibited a substantial increase beginning on the 7th day (Table 1). The inhibition zone further increased to 18.3 mm for V. cholerae and 23.2 mm for S. aureus by day 14, before decreasing to 11.3 and 18.3 mm, respectively, by day 21. The pH decrease observed during fermentation (Figure 1C) could contribute to the increased antibacterial activity, as acidic conditions are known to inhibit the growth of many bacterial pathogens [Sanwal et al., 2023]. Additionally, the phenolic compounds in kombucha are widely recognized for their antimicrobial properties [Nyiew et al., 2022]. Hence, the significant increase in TFC and TPC during fermentation likely contributed to the enhanced antimicrobial activity of PSK. However, we did not observe a consistent correlation between TFC and TPC with antimicrobial activity across three fermentation periods. This suggests that the inhibitory effect of PSK on the pathogenic bacteria may not predominantly rely on its TFC and TPC. Another study also verified the existence of antimicrobial compounds other than organic acids that were generated during fermentation [Mo et al., 2008]. The antimicrobial activity observed in kombucha likely results from a combination of inherent properties from raw materials, as well as organic acids and antimicrobial compounds such as bacteriocins produced by microorganisms during fermentation [Mo et al., 2008; Nyiew et al., 2022; Su et al., 2023]. Further studies are needed to discover the exact mechanisms behind the antibacterial effects of PSK, including the role of pH and the specific compounds involved.
Inhibition of α-amylase activity
The inhibition of α-amylase activity by PSK is a significant area of research, particularly in the context of managing diabetes and obesity. Our previous and other studies have shown that kombucha tea has hypoglycemic effects in both in vivo and in vitro settings [Permatasari et al., 2021; Phan-Van et al., 2024]. Our current data show that inhibition of α-amylase activity of sweetened P. sarmentosum leaf infusion before fermentation was approximately 10.7% (Table 2). By day 7 of fermentation, this inhibition level significantly (p≤0.05) increased to around 13.8%. Although the inhibition percentage increased to 15.2% by day 14 and 15.4% by day 21, there was no significant (p>0.05) difference between days 7, 14, and 21, indicating that the primary increase occurred early in the fermentation process. The inhibitory effect of kombucha on α-amylase is attributed to the combined influence of organic acids formed during fermentation and the enhanced activity of tea-derived compounds activated by the fermentation process [Dickmann et al., 2017]. The findings support the development of PSK as functional food that may aid in preventing conditions related to high blood sugar, such as obesity and hypertension. Given that PSK shows promise as an α-amylase inhibitor, further investigation is required to fully elucidate its effects in animal models.
Sensory evaluation
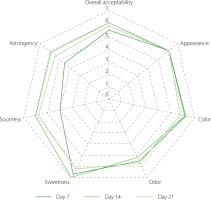
While kombucha is traditionally produced with black tea, using herbal and floral infusions can provide a healthier alternative to sugary soft drinks while also introducing a wider range of flavors [Zhang et al., 2021]. In addition, the sensory profile of kombucha is significantly influenced by fermentation time, which affects perceived turbidity, aroma (including vinegar, citric fruit, and alcoholic notes), and flavor (sour, bitter, and vinegar), highlighting the importance of controlling fermentation duration [Dartora et al., 2023]. In our study, significant changes were observed in the results of the evaluation of sensory attributes of the sweetened P. sarmentosum leaf infusion during the 21-day fermentation period. The optimal fermentation period for achieving a balanced and enjoyable sensory profile in PSK was around 14 days. After this period, there was a decline in sweetness along with a minor decrease in acceptance of sourness and astringency, which influenced overall acceptability (Figure 4). This could be explained by the lower pH value and higher total acidity at 21 days, as shown in Figure 1. Studies have consistently demonstrated that longer fermentations (15–20 days) of yerba maté-based kombucha may lead to higher acidity and a “vinegar taste”, impacting overall sensory acceptance [dos Santos et al., 2024]. Therefore, our findings can guide the production process to ensure a kombucha beverage with high sensory quality.
CONCLUSIONS
The use of P. sarmentosum in kombucha fermentation results in enhanced SCOBY growth, notable pH reduction, increased total acidity, and effective sugar utilization, indicating that P. sarmentosum could serve as an alternative substrate for this process. The significant increase in antioxidant and antibacterial activities, as well as α-amylase inhibition, highlights PSK’s potential as a functional beverage with enhanced health benefits. The sensory evaluation indicates that a 14-day fermentation period was optimal for achieving a balanced and enjoyable sensory profile. In summary, P. sarmentosum shows potential as a raw material for the development of a non-alcoholic functional kombucha beverage, offering promising bioactivities and sensory qualities. Subsequent investigations will examine the physiological effects of PSK in animal models.