INTRODUCTION
In recent times, biodegradable edible coatings have gained significant attention as environmentally friendly alternatives to conventional food packaging. These coatings not only act as a protective barrier against microbial contamination and oxidation but also prevent physical damage. Furthermore, as they are usually made from natural compounds, such as proteins, lipids, and polysaccharides, their use promotes environmental sustainability. By reducing dependence on non-renewable resources edible coatings offer a sustainable approach to postharvest fruit treatment [Miteluţ et al., 2021].
Chitosan is the preferred polysaccharide coating for a range of fruits, mainly due to its ability to minimize moisture evaporation, and reduce browning and fungal infections [Obianom et al., 2019]. Numerous investigations have been conducted to evaluate the potential of chitosan as an edible coating for strawberry fruit, providing both physical and microbial protection [Khodaei & Hamidi-Esfahani, 2019; Saleem et al., 2021]. For example, Jiang et al. [2020] investigated the impact of chitosan coating with varied molecular weights (5, 19, and 61 kDa) on the shelf life of strawberries. A polymer with a molecular weight of 61 kDa substantially decreased respiration rate and water loss in treated strawberries when compared to the untreated fruits.
While the chitosan preservation technique is progressively advancing, innovative approaches, like the addition of natural additives in the coating, remain relatively underexplored. These additives can improve coatings in terms of their antimicrobial and antioxidant properties, thus extending the shelf life of the food product and improving its safety [Priyadarshi et al., 2024]. Martínez-González et al. [2020] formulated a chitosan-based coating incorporating nanostructured chitosan particles and propolis extract. They evaluated its impact on strawberry quality and antioxidant capacity during postharvest storage and found that after 8 days of storage, the coated strawberries exhibited higher contents of total phenolics and total flavonoids, and antioxidant capacitycompared to the uncoated fruits. Another aspect to consider is that the addition of these natural components can amplify the sensory attributes of the coated products, influencing their color, flavor, and aroma, thus making them more appealing to the consumer [Pérez-Santaescolástica et al., 2022]. Furthermore, the use of naturally derived fruit extracts has also shown potential in fortifying edible coatings, particularly in slowing oxidation and controlling enzyme activity [Kumar et al., 2021].
It is estimated that several million metric tons of apple pomace are produced globally each year. Approximately 70–75% of apples are consumed fresh, while the remaining 25–30% is processed into value-added products such as wine, juice, preserves, and dried foods [Plaza et al., 2013]. Unfortunately, a significant portion of apple pomace goes unrecovered, often being discarded as waste. Due to its high content of acids and sugars and low protein content, it is unsuitable for animal feed or landfill disposal. As a result, it is often labelled as food waste. However, apple pomace is rich in nutrients and bioactive compounds, such as carbohydrates, phenolics, dietary fibres, and minerals. The predominant phenolic compounds identified in apple pomace across various cultivars include benzoic acids (such as gallic acid), hydroxycinnamic acids (notably chlorogenic acid), flavan-3-ols ((+)-catechin), flavonols (rutin), and chalcones (phloridzin) [Grigoras et al., 2013]. The phenolic compounds found in apples are recognised for their significant health benefits and their capacity to mitigate oxidative damage caused by free radicals. Therefore, it can be utilised directly or after minimal processing as a functional ingredient in the development of edible coatings [Lyu et al., 2020]. Green techniques for extracting bioactive compounds from apple pomace including minimally processed methods like ultrasound-assisted extraction, cold pressing, supercritical fluid extraction, minimise the use of harmful solvents and energy consumption [Reis et al., 2012]. These environmentally friendly approaches enhance the yield and quality of bioactive compounds while promoting sustainability and reducing waste.
Strawberries (Fragaria × ananassa Duchesne) are hailed as “fruit queen” due to their distinct taste and aroma. They stand out as a highly sought-after fruit across the globe. Their nutritional value is signified by the content of vitamin C, β-carotene, vitamin E, along with other beneficial compounds [Priyadarshi et al., 2024]. However, strawberries have a brief post-harvest lifespan and are highly perishable, primarily due to moisture loss, mechanical injury, and both physical and microbial decay [Riaz et al., 2021].
Given the aforementioned, the application of extract-infused edible coating can simultaneously be used to improve or sustain the nutritional quality of strawberries and contribute to the utilization of the agro-industrial by-product, apple pomace.Although some studies have reported the properties of chitosan-based films with extracts from apple peels and their potential for use in coating strawberries [Riaz et al., 2018, 2021], this research represents the first application of an eco-friendly edible coating made from chitosan infused apple pomace aqueous extract with unique application to fully ripe strawberries.This approach sought to enhance the nutritional quality of strawberries, extend their shelf life, and promote sustainability by utilising an agro-industrial by-product, apple pomace, in a novel and practical application. Fully ripe strawberries have a very limited storage potential period and were selected to test the coating effect in the most challenging conditions.
MATERIALS AND METHODS
Plant material and chemicals
“Albion” strawberries were harvested (22nd May 2023) from the greenhouse of the private company Jagodar-HB in Donja Lomnica, Zagreb County, Croatia. Fruits were immediately transferred to the laboratory. According to visual strawberry maturity detectionbased on additional fruit color development, these strawberry fruits were at the stage of full ripeness and had a very limited storage potential period. This was done intentionally to test the coating effect in the most challenging conditions. Apple pomace was collected from different local apple juice producers in the Zagreb county.High molecular weight chitosan (CAS Number: 9012-76-4, molecular weight 310,000–375,000 Da; viscosity 800–2,000 cP in a 1% (w/w) solution in 1% acetic acid at 25°C)was purchased from Sigma-Aldrich (Saint Louis, MO, USA). All other chemicals were of analytical grade and used as received without further purification.
Ultrasound-assisted apple pomace extraction
Before the preparation of aqueous apple pomace extract, the freeze-dried samples were crushed and sifted using a 0.45 mm metal sieve to ensure uniform particle distribution. Based on the preliminary results and technical simplicity, the aqueous apple pomace extract was prepared by suspending exactly 6 g of apple pomace powder (size <0.45 mm) in 200 mL of distilled water. Ultrasound-homogenization-assisted extraction was performed using UP200St-Sonotrode S26d14 equipment (Hielscher, Teltow, Germany). Amplitude was set to 75% and extraction was carried out for 15 min. The parameters were set based on the preliminary results of the extraction kinetics and the point of inflection of the curve, relative to the highest total phenolic content. Following the ultrasound-assisted process, the extracted solution was filtered through Whatman No. 4 filter paper using vacuum filtration.
Preparation of edible coatings
A chitosan solution (1%, w/v) was prepared by dissolving chitosan in a sterile citric acid solution (2%, w/v) [Saleem et al., 2021]. The solution was stirred and left overnight to ensure full dissolution. The same procedure was used for preparing chitosan infused with aqueous apple pomace extract, which had a total phenolic content of 181.2 mg gallic acid equivalents per L. Citric acid (2%, w/v) was dissolved in the aqueous apple pomace extract. Then, a chitosan solution (1%, w/v) was prepared by dissolving chitosan in the obtained extract solution. The chitosan solution was stirred and left overnight to ensure full dissolution.
Coating application
Two treatments – (i) chitosan coating (chitosan), (ii) chitosan coating infused with apple pomace extract (chitosan/xtr.) – and control (uncoated) were used in this research. Each treatment consisted of three repetitions. Each repetition had 30 strawberries. Before being immersed in the coatings, strawberries were washed using tap water and left to air dry. Three sets (for each treatment) of randomly chosen strawberries (90 fruits per treatment) were submerged in the respective coating solutions for 3 min [Jurić et al., 2023]. After the dipping process, the strawberries were removed from the solutions, well-drained, and left to air dry. Each repetition included one plastic container that is commercially used for strawberry storage. Each container held 30 strawberries. Subsequently, all containers were placed in cold storage conditions (3±1°C, relative humidity of 95%). The samples were examined at intervals of 0, 2, 4, and 9 days.
Weight loss determination
The weight of six fruits per repetition (18 fruits per treatment) was measured on all sampling days using a digital analytical balance (OHAUS Adventurer AX2202, Ohaus Corporation, Parsippany, NJ, USA) with a precision of 0.01 g. Weight loss was calculated according to Equation (1):
where: a is the weight of strawberries at previous inspection day and b is the weight of fruits on the inspection date.Total weight loss was calculated as a difference in weight loss between the first and the last inspection day (9th day).
Firmness, total soluble solids, and titratable acidity measurements
The measurements of firmness, total soluble solids (TSS), and titratable acidity (TA) were performed on five randomly selected strawberries from each repetition (15 fruits per treatment). Firmness was assessed using a PCE PTR-200 penetrometer (PCE Instruments, Jupiter/Palm Beach, FL, USA) equipped with a 6 mm diameter plunger, with results expressed in kg/cm2. Measurements were taken at two opposite equatorial positions on each fruit. TSS was determined using a handheld digital refractometer (Atago, PAL-1, Tokyo, Japan) and expressed in °Brix. TA was measured through titration with 0.1 M NaOH, and results were expressed as g citric acid equivalents per 100 g [Mitcham et al., 1996].
Chemical analysis
Preparation of samples for chemical analysis
Strawberries were crushed and homogenised using a FOSS 2094 equipment (FOSS, Hillerød, Denmark). The resulting homogenates were centrifuged at 1,467×g (9,000 rpm) for 20 min using a NUVE NF 800R multi-purpose centrifuge (NUVE, Ankara, Turkey). The supernatants were then filtered through Whatman No. 4 filter paper and utilised for further analysis, with the juice diluted as needed. Residue after centrifugation was saved for specific chemical analyses. All chemical analyses were performed using a UV-1900i spectrophotometer (Shimadzu, Kyoto, Japan).
Determination of total phenolic content
The total phenolic content (TPC) was determined using a modified spectrophotometric method based on Singleton et al. [1999]. For the analysis, 100 μL of strawberry juice was combined with 7.9 mL of distilled water and 0.5 mL of the Folin-Ciocalteu’s reagent (diluted 1:2, v/v, with distilled water). The mixture was then combined with 1.5 mL of 20% (w/v) Na2CO3 and vortexed thoroughly. After a 2-h incubation, absorbance was measured at 765 nm, and the results were expressed as mg of gallic acid equivalents per L of juice (mg GAE/L).
Determination of total flavonoid content
Total flavonoid content (TFC) was determined using a spectrophotometric method described by Ivanova et al. [2010]. A 1 mL aliquot of strawberry juice was transferred to a 10 mL volumetric flask containing 4 mL of distilled water. To this, 300 μL of an NaNO3 solution (0.5 g/L) was added, followed by 300 μL of AlCl3 (1 g/L) after 5 min. After additional 6 min, 2 mL of an NaOH solution (1 M) was introduced to the mixture. The final volume was adjusted to 10 mL with distilled water. Absorbance was measured at 360 nm, and the results were expressed as mg of quercetin equivalents per L of juice (mg QE/L).
Determination of contents of total anthocyanins and total flavan-3-ols
Total anthocyanin content (TAC) was determined both in the juice and residue after juice separation, while total flavan-3-ol content (F-3-O) only in the residue. Specifically, TAC was determined using 1% (v/v) hydrochloric acid in a 70% EtOH solution. A volume of 0.1 mL of juice was added to the solution (10 mL). For the residue, 0.1000 g was weighed and subjected to solid-liquid extraction using the same solution (10 mL) on an orbital shaker for 20 min. Suspensionswere filteredthrough the Whatman No.4 filter paper and diluted as necessary. Absorbance was measured at 525 nm. The total anthocyanin content was quantified and expressed as mg of cyanidin 3-O-glucoside equivalents (C3G) per L for the juice samples, and per 100 g for the residue samples according to Vieira et al. [2019]. The total flavan-3-ol content was measured using the p-(dimethylamino) cinnamaldehyde (p-DMACA) method [Di Stefano et al., 1989]. In brief, 1 g of the strawberry residue was shaken with 100 mL of an EtOH (70%, v/v)/HCl (1%, v/v) solution for 20 min. An aliquot of 1 mL of the filtrate was added to a 10 mL volumetric flask. Subsequently, three drops of glycerol were introduced, followed by 5 mL of a freshly prepared 1% (w/v) p-DMACA reagent in a chilled mixture of methanol and hydrochloric acid (in a 4:1, v/v, ratio). The total volume was then brought to 10 mL using methanol, and after a 7-min incubation period, the absorbance was recorded at a wavelength of 640 nm. The total flavan-3-ol content was expressed as mg (–)-epicatechin equivalents per 100 g residue (mg EE /100 g).
Determination of antioxidant capacity
The antioxidant capacity of strawberry juice was measured using the DPPH and ABTS assays, which involve 2,2-diphenyl-1-picrylhydrazyl (DPPH) radicals and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) radical cations, following the methods of Brand-Williams et al. [1995] and Re et al. [1999], respectively. The results were expressed as mmol Trolox equivalents per L of juice (mmol TE/L).
Microbiological analysis
Three strawberries from each treatment replicate were smashed and mixed thoroughly inside a sterile stomacher bag. A 10 g aliquot of the blended material was transferred to another stomacher bag, mixed with 90 mL of 0.1% buffered peptone water, and homogenised for 1 min at 250 rpm (BagMixer® 400 P, Interscience, Puycapel, France). In addition, 1 mL of the coating materials was diluted in 9 mL of 0.1% buffered peptone water and homogenised in the same manner as strawberries. Serial decimal dilutions were prepared from these initial dilutions. The Plate Count Agar (PCA; Biolife, Milan, Italy) was used for counting total mesophilic and psychrotrophic bacteria, Violet Red Bile Glucose (VRBG) agar (Biolife) for counting enterobacteria and Dichloran Rose-Bengal Chloramphenicol (DRBC) agar (Biolife,) for yeasts and moulds. The microbial loads were enumerated by spread-plating 100 µL of each dilution into the DRBC and pour-plating 1 mL of each dilution into VRBG and PCA. The aerobic mesophilic count (AMC) was estimated after incubation of PCA Petri dishes for 48 h at 37°C, Enterobacteriaceae were kept for 24 h at 35°C, DRBC Petri dishes were incubated for 7 days at 20°C whereas growth of psychrotrophic bacteria was estimated after incubating PCA plates at 4°C for 7 days [Brasil et al., 2012]. The microbial populations were detected on separate days during the experimental period (9 days), and the results were captured in the form of a log of colony-forming units per g (log CFU/g).
Sensory analysis
Sensory analysis was conducted on day 0 and day 4, given that fully ripe strawberries are generally acquired and consumed within three days post-harvest in commercial settings. Eleven panellists had no previous experience in the assessment of strawberries but were sensory trained. Panellists were of various age groups (22–53 years), genders (8 F and 3 M), and different ethnicities. Panellists were chosen using the following criteria: (a) no strawberry aversions, allergies, or intolerances; (b) no smoking; (c) ages 22 to 65; (d) normal perceptive abilities; (e) availability for all sessions; and (f) interest in participation. The panellists were given a brief explanation to the technique (with question marks). All samples were examined in partitioned booths within the laboratory, with white light illumination at room temperature. Question marks included visual (freshness, color, and glossiness) and organoleptic (hardness, texture, flavor, juiciness and sugar/acid ratio perception) traits with following rating scores: 1 (unsatisfactory), 1.5 (acceptable to unsatisfactory), 2 (acceptable), 2.5 (moderate), 3 (good), 3.5 (good to very good), 4 (very good), 4.5 (very good to excellent), and 5 (excellent). Each panellist was physically separated from the other, and all samples were coded. Firstly, visual attributes were scored based on the whole strawberry and afterward, organoleptic traits were scored based on strawberry slices. Between each tasting, water and white bread were provided for the taste neutralisation.
Statistical analysis
The obtained dataset was analyzed using IBM SPSS Statistics 22 (IBM Corp., Armonk, NY, USA) and XLSTAT add-on (Addinsoft, Paris, France) for Microsoft Office 2016 (Microsoft, Redmond, WA, USA). An analysis of variance (ANOVA) was employed for results of chemical, sensory and microbiological analyses to determine differences between treatments and storage times. The chemical analysis data are represented as means with standard deviations obtained from three replicates. The significance (p<0.05) was established using the post hoct-tests with Bonferroni adjustment. The microbiological data are shown as means with standard deviations from three replicates. Before statistical analysis, the normal distribution of microbiological data was assessed using the Shapiro-Wilk test, and the homogeneity of variance using Levene’s test. Given the non-normal distribution and heterogeneous variance of the data, statistically significant differences in microbial purity of the edible coatings were determined using the Kruskal-Wallis test, while the microbial counts of different microbial groups on strawberries were analyzed using the Scheirer-Ray-Hare test. To determine statistically significant differences between the groups, the multiple pairwise comparisons were performed using post-hoc Dunn’s test with a Bonferroni adjustment of p-values. Differences with p<0.05 were considered statistically significant.
RESULTS AND DISCUSSION
Ultrasound-assisted extraction of apple pomace
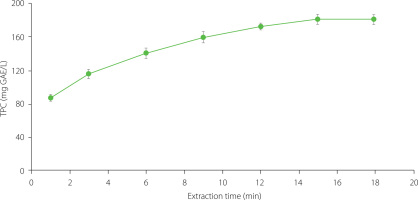
Minimising food loss and waste is essential for sustainable development, and hence shelf-life extension technologies are exploring innovative solutions. This research utilised aqueous apple pomace extract obtained via ultrasound-assisted extraction to enrich a 1% chitosan solution for the development of edible coatings on fully ripe strawberries. Ultrasound-assisted extraction is a non-thermal process that applies acoustic energy to increase the release and diffusion rates of bioactive compounds by breaking down the cell walls via cavitation of the solvent [Misra et al., 2018]. In the preliminary experiment, the ultrasound-assisted aqueous extraction time of apple pomace was optimised to achieve the highest content of phenolic compounds in the extract The optimal extraction time for the highest yield of phenolic compounds was 15 min, as shown in Figure 1. Finally, the total phenolic content of extract was 181.2 mg GAE/L corresponding to 6.04±0.20 mg GAE/g apple pomace powder.
Weight loss, firmness, total soluble solids, and titratable acidity of strawberries
Through this research, chitosan, a natural biodegradable polysaccharide extracted from marine natural sources, was used as an edible coating, while an aqueous apple pomace extract was used to potentially enhance edible coating performance. The application of edible coatings did not have a significant effect (p≥0.05) on any of the investigated fruit quality parameters (weight loss, firmness, TSS, and TA) throughout the storage (Table 1). Total weight loss varied from 21.05% for the control group, 22.15% for the chitosan/xtr. treatment, to 22.26% for the chitosan-coated strawberries. Possible explanations for the non-significant effect of the coatings may be attributed to the relatively brief duration of storage for fully ripe strawberries. Similar results were found previously where from day 3–6, the weight loss of coatings-treated strawberries showed no significant differences as compared with the control [Liu et al., 2021]. Furthermore, in the same study, it is worth noting that after longer periods of storage (8 days), a coating composed of the hydroxyethylcellulose/sodium alginate blend infused with asparagus waste extractsignificantly decreased weight loss. Nevertheless, the treatment with only hydroxyethylcellulose infused with extract significantly increased the weight loss of strawberry fruit [Liu et al., 2021]. These results highlight the importance of composition when preparing blends of biopolymers as coating materials. Also, in this case, it can be observed that the weight loss is primarily related to the edible coating and not the additive (i.e., natural extract). Generally, the weight loss of fresh strawberries is mainly due to the water loss. This can be attributed to the varied pore sizes and pore numbers present in the coatings, influencing the moisture and gas exchange.
Table 1
The weight loss, firmness, total soluble solids (TSS), and titratable acidity (TA) of untreated strawberries (control), strawberries coated with chitosan (chitosan), and strawberries coated with chitosan/apple pomace extract (chitosan/xtr.) over storage time (0–9 days).
There was no significant difference (p≥0.05) recorded regarding the treatment effect on strawberry firmness or TSS levels. Similarly, an outcome was observed before, where chitosan coating infused with apple peel polyphenols was applied [Riaz et al., 2021]. Related to TSS, another study on quality attributes and sensory tests [Azodanlou et al., 2003], reported that strawberries were always highly appreciated if their sweetness and aroma intensity were high. In the cited study strawberries that had on average 8.3ºBrix belonged to a very good quality class in contrast to those with lower TSS values. Accordingly, it can be considered that strawberries used in this study had excellent TSS levels for consumer appreciationand that their TA levels were around the maximum proposed by Kader [2005] who found that strawberries should have minimum of 7ºBrix SSC and maximum of 0.8% TA. In this study TA levels were around or slightly above the proposed aforementioned maximum. Slightly and partially alleviated TA levels do not indicate not-optimal taste, since SSC levels were much higher than the proposed ones. The primary organic acids in strawberry fruit are citric and malic acids, which are important flavor compounds [Azodanlou et al., 2003]. Although no significant differences were recorded, TA was elevated in chitosan/xtr. treated strawberries in contrast to the control ones on day 9 of cold storage (Table 1). Moreover, on the last sampling day, TA decrease was highest in the control and lowest in the chitosan/xtr. samples. A similar finding was reported by [Riaz et al. 2021], who observed greater differences in strawberries coated with apple peel polyphenol-infused chitosan (0.78%) when compared to only chitosan coating (0.68%).
Bioactive compound content and antioxidant capacity of strawberries
Bioactive compounds are part of the natural plant defence mechanisms. Specifically, phenolics are organic compounds that have gained significant attention in the nutritional domain over the past years. An increasing number of studies suggest that the intake of phenolics might be crucial for health, influencing metabolism, weight management, chronic illness prevention, and cellular growth [Cory et al., 2018]. The effects of coating on the strawberry’s total phenolic content and total flavonoid content are depicted in Figure 2. Interestingly, juice from chitosan/ xtr. treated strawberries had significantly (p<0.05) higher TPC, relative to the control after 2, 4, and 9 days of storage. On days 4 and 9, both coatings (chitosan and chitosan/xtr.) significantly (p<0.05) influenced TPC in juice when compared to the control. Respectively, the TPC showed an increasing trend over storage time in the treated samples. Similar findings in TPC were reported before where authors utilised chitosan infused with apple peel polyphenols as a coating for strawberry fruits [Riaz et al., 2021]. Fagundes et al. [2013] suggested that tomato fruit coated withhydroxypropyl methylcellulose-lipid exhibited an increased phenolic content over time. Liu et al. [2021] also observed an increase in the total phenolic content at the end of an 8-day storage period (25°C) in strawberry fruits coated with hydroxyethyl cellulose incorporating asparagus waste extract. It has to be noted that there are some contradictory results reported, where strawberry fruits coated with chitosan-based coatings and carboxymethyl cellulose show a decreasing trend in TPC during the storage period [Khodaei & Hamidi-Esfahani, 2019].
Figure 2
Total phenolic content (TPC) (A) and total flavonoid content (TFC) (B) of untreated strawberries (control), strawberries coated with chitosan (chitosan), and strawberries coated with chitosan/apple pomace extract (chitosan/xtr.), over storage time (0–9 days). Values marked by a different letter within the same sampling time are significantly different (p<0.05) according to the post hoc t-tests with Bonferroni adjustment. GAE, gallic acid equivalent; QE, quercetin equivalent.
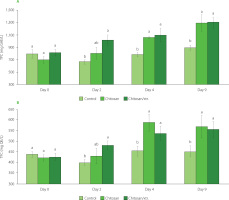
A similar trend to the TPC was observed for TFC (Figure 2B). It was previously reported that chitosan coatings effectively stimulate the production of flavonoids in mandarin fruits [Jurić et al., 2023]. This stimulation might be a reaction to the direct application of the acidic chitosan solution onto the surface of mandarin fruit. Such an environmental change (introduction of an acidic medium) may trigger the fruit to enhance its flavonoid biosynthesis, leading to elevated flavonoid levels after storage. Herein, on day 4 and day 9, juices of the treated strawberries had significantly (p<0.05) higher TFC than the control (Figure 1B). Again, similarly to the TPC, on the second sampling day, the difference was significant (p<0.05) only for juice of the chitosan/xtr. treated strawberries. In addition, the TFC of the control during storage showed a trend without change. Herein we report that at the end of storage, both coatings (chitosan and chitosan/ xtr.) resulted in significantly higher TFC in strawberry juices with values of 568.3 mg QE/L and 556.9 mg QE/L, respectively. However, the highest TFC (587.9 mg QE/L) was determined for the chitosan-coated strawberries at day 4 of storage. The findings regarding total phenolic and total flavonoid contents further align with the observations made by Liu et al. [2021]. They noted that hydroxyethyl cellulose coatings might have difficulties forming dense layers because of their inherently porous structure. Conversely, coatings like hydroxyethyl cellulose/sodium alginate blend and sodium alginate proved more effective in forming barriers against oxygen, subsequently slowing down the deterioration of phenolics, including flavonoids, in strawberries.
Anthocyanins, water-soluble pigments, typically located in plant cell vacuoles, belong to the group of flavonoids. The red color of strawberries is due to the presence of anthocyanin pigments. In strawberry fruits, the main anthocyanins include pelargonidin 3-glucoside, while pelargonidin 3-rutinoside and cyanidin 3-glucoside are present as minor compounds [Petriccione et al., 2015]. In this work, TAC was spectrophotometrically analyzed, both, in the strawberry juice (Figure 3A) and the residue after juice separation (Figure 3B). TAC in juice increased during storage for both treatments, while the same was not the case for the control. On the initial day, TAC in juice ranged from 213.1 mg/L to 217.8 mg/L. At the end of storage, total anthocyanin content increased for the strawberries treated with chitosan and chitosan/xtr. to 292.1 and 259.4 mg/L, respectively, and decreased for the control to 207.20 mg/L. Riaz et al. [2021] investigated the efficacy of chitosan-based coatings with added apple peel polyphenols during strawberries storage, and observed a reduction in the anthocyanin content of the coated fruits. A noticeable uptick in anthocyanin content was observed in the extract-infused chitosan-treated strawberries on the second day, which might be attributed to the ongoing production of these compounds post-harvest [Sogvar et al., 2016]. As reported by Riaz et al. [2021], throughout storage, a decline in anthocyanin levels in treated strawberries (after day 2) might result from the increased enzyme activity, specifically polyphenol oxidase. The composite coatings functioned as a barrier to gaseous exchange, potentially altering fruit biochemical processes responsible for anthocyanin production. However, this was not the case in our work where TAC in juice increased after 4 and 9 days of storage and only for the treated strawberries. This can be attributed to the fact that only one biopolymer (chitosan) was used and not a blend (combination of biopolymers), signifying the importance of coating composition and porosity which should be finely regulated if the goal is to increase anthocyanin content. Considering the TAC in the residue (Figure 3B), a similar trend was observed as in juice, except on day 2 of storage where relatively highest values were recorded for all samples.
Figure 3
Total anthocyanin content (TAC) in juice (A), TAC in residue after juice separation (B), and total flavan-3-ols content (F-3-O) in residue (C) of untreated strawberries (control), strawberries coated with chitosan (chitosan) and strawberries coated with chitosan/apple pomace extract (chitosan/xtr.), over storage time (0–9 days). Values marked by a different letter within the same sampling time are significantly different (p<0.05) according to the post hoc t-tests with Bonferroni adjustment. C3G, cyanidin 3-O-glucoside equivalent; EE, (−)-epicatechin equivalent.
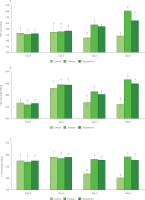
Flavan-3-ols are common compounds found in seeds of strawberry fruit. In this study, the content of flavan-3-ols was determined in strawberry residue (Figure 3C). On day 0 and day 2, there were no significant differences (p≥0.05) between treatments in the content of total flavan-3-ols. Similarly, as for other recorded parameters (TPC, TFC, TAC, antioxidant capacity), at day 4 and 9, the residue of the treated strawberries maintained a significantly higher total flavan-3-ol content than the control. The content of flavonoids (flavan-3-ols and anthocyanins) generally decreases due to oxidation reactions during storage, and the application of chitosan coating maintained flavan-3-ol levels due to the reduction in oxygen availability [Saleem et al., 2021].
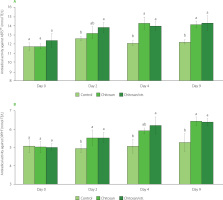
Regarding the antioxidant capacity of strawberries determined with the ABTS and DPPH assays, we have obtained a high correlation with a correlation coefficient (r) of 0.89 between the methods. The antioxidant capacity of the treated strawberries was found to be significantly higher than of the control on day 4 and day 9 (Figure 4). It is also worth noting that the results of ABTS assays for only chitosan/xtr. treated strawberries were significantly (p<0.05) higher than for the control on day 2, while chitosan-only treated strawberries did not significantly differ (p≥0.05) in their antioxidant capacity (both assays) at that time. Generally, antioxidant capacity of strawberries highly correlates with the total phenolic content and total flavonoid content [Chen et al., 2023], which is following the results presented in this study. Furthermore, Tulipani et al. [2008] have linked antioxidant capacity of different strawberry genotypes with the presence of effective oxygen-radical scavengers, such as phenolic compounds and ascorbic acid. Antioxidants were relatively stable in the coated fruits as compared to the uncoated ones. The coating helps reduce enzyme activity and preserve the fruit quality, which slows down the breakdown of antioxidant compounds, especially those that act as effective oxygen radical scavengers [Riaz et al., 2021].
Figure 4
Antioxidant capacity of juices from untreated strawberries (control), strawberries coated with chitosan (chitosan), and strawberries coated with chitosan infused with apple pomace extract (chitosan/xtr.) determined using ABTS assay (A) and DPPH assay (B) over storage time (0–9 days). Values marked by a different letter within the same sampling time are significantly different (p<0.05) according to the post hoc t-tests with Bonferroni adjustment. TE, Trolox equivalent.
Microbiological quality of strawberries and coatings
The AMC and yeast and mould count in the untreated and the coated strawberries are shown in Table 2. Yeasts and moulds were not detected in the coating materials, while AMC was 3.97±2.78 and 4.27±2.42 log CFU/g in chitosan and chitosan/xtr., respectively. Enterobacteriaceae and psychrotrophic bacteria were not detected in any of the samples tested.
Table 2
The aerobic mesophilic count (AMC) and yeast and mould count (log CFU/g) of untreated strawberries (control), strawberries coated with chitosan (chitosan), and strawberries coated with chitosan/apple pomace extract (chitosan/xtr.) over storage time (0–9 days).
In the untreated fruit (control), AMC was constant and ranged from 4.45 log CFU/g (day 0) to 4.47 log CFU/g (day 9), while yeast and moulds were present only in the control samples during the first two days of storage (2.82 log CFU/g for day 0, and 2.94 log CFU/g for day 2) (Table 2). Contrary to the expected increase in AMC, yeast, and mould counts typically observed in untreated strawberries during storage [Khan et al., 2019; Martínez et al., 2018], in our study, AMC remained stable throughout the storage, while yeasts and moulds could no longer be detected in the uncoated strawberries after the second day. The absence of yeasts and moulds can be attributed to several factors. Firstly, the low initial yeast and mould loads on strawberries at the beginning of the experiment, which may be explained by the low contamination levels during the ripening and harvesting of the strawberries, as these phases determine the post-harvest fungal incidence [Contigiani et al., 2018]. Furthermore, the storage conditions similar to those used in our experiment (3°C, humidity level between 90 and 95%) have been reported to slow down the metabolic activity of yeasts and moulds, lowering their proliferation, and reducing spore viability, thus reducing overall fungal counts [Ansiska et al., 2023; Feliziani & Romanazzi, 2016; Trinetta et al., 2020]. Additionally, the bioactive compounds inherent in strawberries, mainly various phenolic compounds with antifungal activity, can effectively inhibit the mycelial growth and slow down the spore germination [El-Seedi et al., 2012; Morales et al., 2017; Pott et al., 2020], further reducing fungal counts. Finally, competition for niche and nutrients with other microorganisms that are part of natural strawberry microbiota [Sretenović et al., 2024], may also account for the absence of yeast and moulds and the sustained stable AMC.
The increased AMC in coated strawberries compared to the control at day 0 was most likely associated with the bacteria introduced by the coating and extracts. Although chitosan and chitosan-based edible polymers are known to be antimicrobial [Khan et al., 2019], they can also be sources of microorganisms, which was mostly not considered in previous studies. Despite the introduction of microorganisms by the coating materials, the AMC and level of yeasts and moulds in the coated strawberries in our study was lower than in the control samples, which is in agreement with the study by Khan et al. [2019] on chitosan-based edible coatings for strawberries.
Later in the storage period, a decrease in AMC was observed in the chitosan-coated fruit (from 5.37log CFU/g, day 0, to 3.54log CFU/g, day 9) and in the chitosan/xtr. fruit samples (from 4.51log CFU/g, day 0 to 4.12 log CFU/g, day 9) (Table 2). The AMC was significantly lower in the coated strawberries compared to the uncoated ones, regardless of the type of coating and the storage time (Dunn’s test with Bonferroni correction, p<0.05). While chitosan tended to suppress AMC more effectively than chitosan/ xtr. for most of the 9-day storage period, this difference was statistically significant only at day 9, suggesting that the efficacy of chitosan in suppressing AMC becomes more pronounced later in the storage period. Yeasts and moulds were not detected in the coated fruit samples (<1 log CFU/g), regardless of the day of storage. The antimicrobial effect observed in this study is most likely due to the antimicrobial properties of chitosan, which is consistent with previous studies on strawberries treated with chitosan-based edible coatings [Khan et al., 2019; Martínez et al., 2018]. While phenolic compounds from apple pomace are also known for their antimicrobial properties [Riaz et al., 2018; Wang et al., 2015], our results suggest that the addition of apple pomace extract to chitosan coating did not significantly improve the microbiological quality or shelf-life of the strawberries compared to the chitosan coating alone.
Regardless of the day of storage, no visible signs of defect were observed on any of the fruit analyzed. AMC was generally 3–4 log lower than the value of 7.0–8.0 log CFU/g suggested by Ragaert et al. [2006] as critical for organoleptic alteration in foods.
Our results indicate a good microbiological quality of the tested fruit and are in agreement with the results of studies on the microbiological quality of berries in the EU and the USA [Macori et al., 2018; Quansah et al., 2019]. For example, the AMC ranged from 1.7 to 6.9 log CFU/g in most of the samples analyzed by Macori et al. [2018] and averaged 3.89 log CFU/g in the study by Quansah et al. [2019]. Enterobacteriaceae were detected in the range of 1 to 4 log CFU/g in 13% of the samples analyzed by Macori et al. [2018], and the total yeast and mould counts and the total coliform counts in the berries studied by Quansah et al. [2019] were 4.42 and 1.42 log CFU/g, respectively.
Overall, our results have shown that chitosan-based edible coatings represent an effective and sustainable approach for controlling the growth of undesirable microbiota and extending the shelf-life of strawberries while preserving organoleptic properties during storage.
Sensory evaluation of strawberries
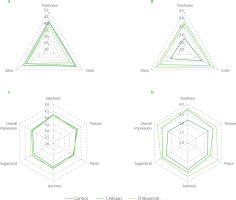
Sensorial characterisation plays an important role in consumer food acceptance [Khodaei et al., 2021]. Sensory characteristics of the strawberries were evaluated on 0 and 4 days of the study, and the results are presented in Figure 5. Initially, the highest score in freshness was given to the strawberries coated with chitosan/apple pomace extract, while the lowest color score was given to the chitosan-coated strawberries. Regarding the organoleptic parameters, juiciness, sugar/acid perception, and overall impression were rated the lowest for the control strawberries. However, differences in terms of values were negligible. On day 4, interestingly, the relatively lowest visual parameters (gloss, color, and freshness) were found in the control samples. Hence, both treated strawberries can be characterised by positively increased visual perception. The significant increase in the total anthocyanin content in the coated strawberries during storage life may explain the preference of the panellists for these samples. Since at the time of initial purchase consumers judge the quality of the fresh fruit based on appearance (including “freshness”) [Kader, 2005], this may have a crucial role in the customer’s decision to buy strawberries. The relatively lowest organoleptic scores (hardness, texture, flavor, juiciness, and sugar/acid ratio perception) were noted at day 4 for the control samples, whereas the treated strawberries showed superiority. The sensory attributes of strawberries that have undergone treatment are crucial from an economic standpoint for producers. This is because repeat purchases are largely contingent on consumer contentment, particularly concerning the taste and overall eating quality of the product. Ensuring that the strawberries meet or exceed consumer expectations in these areas can significantly influence their market success and profitability.
Figure 5
Sensory evaluation of untreated strawberries (control), strawberries coated with chitosan (chitosan), and strawberries coated with chitosan infused with apple pomace extract (chitosan/xtr.) in terms of visual impressions at day 0 (A) and day 4 (B), as well as in terms of taste on day 0 (C) day and day 4 (D).
CONCLUSIONS
The developed chitosan and apple pomace extract-infused chitosan edible coatings improved, maintained, and effectively enhanced the bioactive properties, sensory attributes, and microbiological quality of fully ripe strawberries during storage. The study demonstrates that these coatings increase the total phenolic content, the total flavonoid content, and antioxidant capacity while reducing microbial counts. Additionally, the coatings maintain the strawberries’ visual and organoleptic quality, extending their shelf-life in a sustainable and eco-friendly manner. Throughout the 9-day storage period, the fully ripe strawberries exhibited no significant variations in weight loss, firmness, the total soluble solids, or titratable acidity. No significant differences were observed between the chitosan coating and the extractinfused chitosan coating in terms of the total phenolic content, the total flavonoid content, and antioxidant capacity in strawberries after 4 days of storage. Conversely, on day 2, the extractinfused chitosan coating exhibited a significant impact, leading to increased levels of total phenolic compounds and flavonoids, and antioxidant capacity when compared to the plain chitosan coating. Moreover, both chitosan and apple pomace extractinfused chitosan edible coatings effectively suppressed microbial growth in strawberries, and significantly reduced AMC compared to the untreated controls over a 9-day storage period. In addition, no yeasts or moulds were detected in the coated samples during the entire storage period. While the addition of apple pomace extract did not significantly enhance antimicrobial efficacy of chitosan, chitosan-based edible coatings offer a promising approach for preserving strawberry quality and extending shelf-life. Herein we point out that there are some limitations to this research work. One of them is the relatively low bioactive content (total phenolic content) in the initial edible coating due to the simple ultrasound-assisted extraction method with the use of only water as a solvent. This can be further mitigated by applying different green extraction techniques and green solvents to achieve a higher initial content of bioactive compounds. Overall, chitosan proved its potential in various studies as an edible coating, and here its influence was confirmed. However, there is still a need for additional research dealing with chitosan concentration, application of additives and preparation of adequate blends (composites) with desirable porosity and functionality.