ABBREVIATIONS
ABTS•+, radical cation of 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid); ANOVA, analysis of variance; DPPH•, 2,2-diphenyl-1-picrylhydrazyl radical; FTIR, Fourier transform infrared spectroscopy; GAE, gallic acid equivalents; GDRI, glucose dialysis retard index; IDF, insoluble dietary fiber; OAC, oil absorption capacity; SEM, scanning electron microscopy; RE, rutin equivalents; SDF, soluble dietary fiber; TDF, total dietary fiber; WAC, water absorption capacity.
INTRODUCTION
The agro-industrial by-products generated during the processing of fruits and vegetables are potential sources of bioactive compounds, such as carotenoids, phytosterols, phenolics, and dietary fiber [Lemes et al., 2022; Reguengo et al., 2022]. In this context, some authors have focused their attention on the study of by-products obtained from fruits and vegetables as sources of dietary fiber (DF) [Garcia-Amezquita et al., 2018]. According to Gan et al., [2021], the ultimate purpose is to apply the DF in the functional food industry. Specifically, DF is associated with the reduced risks of coronary heart disease, type-2 diabetes, inflammatory bowel disease, constipation, and colon cancer [Gustafson & Rose, 2022; Martínez-Flores et al., 2009; Yusuf et al., 2022]; these beneficial physiological effects of DF are directly related to its physicochemical and technological properties, such as water retention capacity, swelling capacity, viscosity, and the ability to bind bile salts and organic molecules such as glucose and lipids [Elleuch et al., 2011; Larrea et al., 2010]. Phenolics, other phytochemicals present in fruits and vegetables, are antioxidants that delay or inhibit the oxidation of molecules in the human body and, therefore, reduce the risk of oxidative stress and diseases such as cancer, diabetes, and cardiovascular disease [Kumar & Goel, 2019].
Relevant to this study, the tamarind plant (Tamarindus indica L.) is a multipurpose tree whose roots, wood, leaves, fruits, seeds, and pod shells are used to make food items, drugs, herbal medicine, fuel, timber, fodder, and textile industry products [Chimsah et al., 2020; De Caluwé et al., 2010]. Upon the separation of the tamarind fruit, the residues – constituted by the seed and the shell – that remain are considered as its by-products. The tamarind shell is used as a biosorbent to remove chemical substances, such as malachite green [Saha et al., 2010], and to produce briquettes [Ujjinappa et al., 2018] and tannins, the latter being used as potential green rust converter [Abdulmajid et al., 2019]. Another potential use of the tamarind shell entails the modification of its properties, particularly by modification of its DF, to obtain new functional characteristics [Gan et al., 2019]. Some investigations have focused on the functional and physicochemical changes of the DF generated by the extrusion process [Benítez et al., 2023; Huang et al., 2019; Zhang et al., 2011; Zhong et al., 2019]. The tamarind shell is characterized by the presence of mostly insoluble dietary fiber (IDF) and very little soluble dietary fiber (SDF). Thus, a thermomechanical modification by extrusion, that converts IDF to SDF, could be a viable alternative for obtaining a product designed for application purposes in the food industry [Menis-Henrique et al., 2020]. Barak & Mudgil [2014], mentioned that soluble fiber, such as locust gum bean, was used as a thickener, stabilizer and gelling agent in various food products, such as baked foods, beverages, dairy products, and processed fruit products. Increasing the amount of soluble fiber in a sample, could be useful in products that require greater water absorption capacity, for example, in preventing syneresis in products such as yogurt [Mudgil, 2018].
Unfortunately, there is a lack of research addressing the application of the extrusion technology to the tamarind shell. Therefore, the objective of this study was to apply the extrusion process to modify some physicochemical properties of the tamarind shell without affecting bioactive compounds such as phenolics, as well as their antioxidant capacity.
MATERIALS AND METHODS
Raw material
Ten kilograms of tamarind shells meant for extrusion were obtained from the company La Estrella® located in the city of Morelia, Michoacán, Mexico. Tamarind shells were oven-dried at 40°C (FE-292 oven, Felisa®, Zapopan, Jalisco Mexico) for 48 h and crushed in a Pulvex model 200 mill (Molinos Pulvex, S.A. de C.V., México city, Mexico) using a mesh of 800 μm. The tamarind shell samples were blended with water for 10 min using a mixer (Kitchen Aid model K45SS, St. Joseph, MI, USA) until reaching a moisture content of 32 g/100 g and 39 g/100 g. Samples were placed at 4°C, in Ziploc® hermetic bags (SC Johnson and Son Inc., Racine, WI, USA) for 12 h until they were processed by extrusion.
Extrusion of tamarind shells
The crushed tamarind shells with moisture content of 32 g/100 g or 39 g/100 g were extruded using and extruder designed and built in the Mechatronics Laboratory of the Center for Research in Applied Science and Advanced Technology, National Polytechnic Institute (CICATA-IPN), Querétaro, Mexico. It consisted of a single screw with a ratio of length to diameter of 21, a channel length of 18 mm, and a channel depth of 3 mm. The output die was a metal plate with a 6 mm diameter hole. The extruder had two heating zones: zone one was maintained at a constant temperature of 60°C and zone two was set at temperatures of 90°C, 100°C and 110°C, respectively, according to previously established conditions. Additionally, the extruder allowed speed control by means of a Baldor VS1 inverter at 15 RPM. A complete factorial design was used, with two factors: temperature and moisture content, the first with three levels (90°C, 100°C and 110°C) and the second with two levels (32 g/100 g and 39 g/100 g), with a total of six treatments. An amount of 250 g of sample was used in each treatment, with two repetitions per treatment. The extrudates were stored at 4°C in sealed bags until analysis. The non-extruded tamarind was taken as a control.
Proximate and dietary fiber composition analyses
Chemical analyses were performed on the non-extruded sample and the extrudates, i.e., tamarind shells with different moisture contents extruded under different temperatures. The proximate composition of the non-extruded samples was determined by the American Association of Cereal Chemists (AACC) methods [AACC, 2000], for contents of moisture (AACC method 44-19), ash (AACC method 08-01), crude protein (AACC method 46-13), and fat (AACC method 30-25). The soluble, insoluble, and total dietary fiber contents in tamarind shell and extrudates were determined by the method of Prosky et al. [1988]. The pectin level in tamarind shells was quantified using the method recommended by Carré & Haynes [1922]. Briefly, 5 g of sample was added to 100 mL of distilled water, the blend was boiled three times, filtered, and in each filtrate the volume was recovered by adding 100 mL of water, having a final volume of 400 mL. An amount of 100 mL of 0.1 M sodium hydroxide were added to the filtrate, which was left to stand for 12 h. Subsequently, 50 mL of 1 M acetic acid were added, and the mixture was allowed to settle for 5 min to finally add 50 mL of 1 M calcium chloride and allowing the mixture to settle again for 1 h; after which it was boiled for 5 min and filtered. After the residue was washed with 500 mL of hot distilled water to remove chlorine, the obtained precipitate was redissolved in 100 mL of distilled water at 25°C and boiled for 5 min. Finally, it was filtered on open-pore filter paper previously set to constant weight, the paper with the residue was washed and dried until constant weight. The pectin content was expressed in g per 100 g of dry matter (DM) of tamarind shell.
Extractable-free samples were obtained to determine lignin, cellulose and hemicellulose contents. Briefly, 5 g of the non-extruded and extruded dried tamarind shells were placed in cellulose cartridges in the Soxhlet equipment, 150 mL of cyclohexane were added to a balloon flask and refluxed for 6 h of extraction. After, the equipment was allowed to cool, it was disassembled and the cartridges were left to air overnight. The same procedure was carried out with acetone, methanol, and water. Finally, the sample was spread on a filter paper to dry at room temperature overnight.
The lignin content was estimated according to the Runkel & Wilke [1951 method. Briefly, 2 g of extractable-free samples from non-extruded tamarind shells or extrudates were weighed and placed in a 200 mL Erlenmeyer flask; then, 50 mL of 72% sulfuric acid, 5 mL of 49% hydrobromic acid were added and left to settle for 2 h in an extraction hood. After this time, 200 mL of cold distilled water were added to stop the reaction, and the mixture was boiled for 5 min, filtered and rinsed with hot water until a neutral pH. The sediment with lignin was left to dry on a glass Petri dish in an oven at 105°C for 6 h, tempered and weighed. The lignin content (g/100 g DM) was calculated on the basis of the mass balance.
Before cellulose determination, extractable-free samples from non-extrude material and extrudates were delignified by the method of Wise et al. [1946]. Five g of extractable-free samples were placed in a 200 mL Erlenmeyer flask, to which 1.5 g of sodium chlorite, 160 mL of distilled water and 10 drops of acetic acid were added. The flask was covered with aluminum foil and placed in a water bath at 75°C for 1 h. After this time 1.5 g of sodium chlorite and 10 drops of acetic acid were added again. This procedure was repeated two more times until 4 h of digestion was completed. Upon completion, the reaction was stopped by placing the flask in an ice bath and when it cooled, it was filtered and washed with cold water and acetone, dried in an oven at 40°C for three days, and weighed. The cellulose content was determined according to the American National Standards Institute (ANSI) standard method D1103-60 [ANSI/ASTM, 1977]. To this end, 2 g of holocellulose extracted from non-extruded and extrudates samples were added with 10 mL of 17.5% sodium hydroxide at room temperature. The mixture was left to stand for 5 min and another 5 mL of NaOH were added, and the mixture was again left to stand for 5 min. The procedure was repeated once more to finally let it rest for 30 min. Then, 33 mL of distilled water were added at room temperature, and the sample was left to rest for 1 h. After filtration in a Gooch crucible, sediment with cellulose was washed with 35 mL of 8.3% NaOH solution distilled water and 10% acetic acid, allowed to stand for 3 min, dried in an oven overnight at 105°C, and weighed. The results were expressed as g of cellulose per 100 g of DM of tamarind shell or extrudate.
The hemicellulose content was obtained by difference, by subtracting the holocellulose content minus the cellulose content, and was expressed as a g of hemicellulose per 100 g of DM of tamarind shell or extrudate.
Glucose dialysis retardation index determination
The glucose dialysis retardation index (GDRI) was determined according to the method described by Adiotomre et al. [1990] with some modifications. Ten milliliters of a 50 mM (900 mg/dL) glucose solution containing 0.2 g of each sample were taken, these were placed in dialysis cellulose bags 11 cm long. Each sample was previously hydrated in the glucose solution for 45 min. Each bag was closed and suspended in 100 mL of distilled water and then placed in a constantly shaking bath at 37°C for 4 h. A 2 mL aliquot was taken to measure its glucose concentration by means of the glucose oxidase method with a Randox (Crumlin, County Antrim, UK) brand kit at a time of 0, 10, 20, 30, 60, 120, 150, 180, 210 and 240 min. GDRI of non-extruded tamarind shell and its extrudates was calculated according to Liu et al. [2018] using the formula (1):
where: GC, glucose content.Determination of water and oil absorption capacities
The water absorption capacity (WAC) and oil absorption capacity (OAC) were determined according to the methodology proposed by Anderson et al. [1969]. To WAC determination, 1 g of non-extruded tamarind shell and extrudates samples were weighed in a Falcon tube, and 10 mL of distilled water was added. They were left to rest for 30 min, shaking every 30 s. Subsequently, suspensions were centrifuged at 670×g for 10 min (Z 400 K centrifuge, Hermle AG, Gosheim, Germany). The supernatant was discarded, and the solid residue was weighed. The amount of water absorbed was calculated by measurement of difference in the weights of the samples before and after centrifugation. The results were expressed in terms of DM of tamarind shell and extrudates. The same procedure was used with the replacement of distilled water with soybean oil to determine OAC.
Extraction of bioactive compounds
An aliquot of 10 mL of methanol was added to 1 g of each non-extruded an extrudates samples, and then the samples, protected from light, were stirred for 24 h. Subsequently, they were centrifuged using a Hermle Z 400 K centrifuge at 2,680×g for 10 min. Thereafter, the extracts were kept at 4°C until they were used to determine the total phenolic content, total flavonoid content, and antioxidant capacity.
Total phenolic content determination
Total phenolic content of non-extruded tamarind shell and extrudates was determined using the Folin-Ciocalteu’s reagent by the method of Singleton & Rossi [1965]. The absorbance was measured at 765 nm on a UV/Vis Smartec Plus spectrophotometer (Bio-Rad, Philadelphia, PA, USA). The results were expressed as mg of gallic acid equivalents per g of DM of non-extruded and extruded samples (mg GAE/g DM).
Total flavonoid content determination
Total flavonoid content of non-extruded tamarind shell and extrudates was determined using the method described by Oomah et al. [2005]. A total of 200 μL of the extract from the tamarind shell or extrudates were reacted with 80 μL of 2-aminoethyl diphenylborate and 720 μL of distilled water. The mixture was incubated for 30 min in the dark, and absorbance was read at 404 nm. Rutin was used as standard. The results were expressed as mg of rutin equivalents per g of DM of sample (mg RE/g DM).
Determination of DPPH• scavenging capacity
The ability of tamarind shell and extrudates to scavenge the 2,2-diphenyl-1-picrylhydrazyl radical (DPPH•) was determined using the method described by Brand-Williams et al. [1995]. The DPPH• was dissolved in methanol until an absorbance of 0.75–0.78 at 517 nm was obtained (using UV/Vis Smartec Plus spectrophotometer, Bio-Rad). Then, 50 μL of each extract was added to 2.95 mL of DPPH• solution, mixtures were shaken and kept in a dark space at room temperature for 30 min. Thereafter, absorbance (Ae) was read at 517 nm. A blank sample containing methanol instead of extract was prepared, and its absorbance (Ab) was measured. The percentage of DPPH• inhibition was calculated using the formula (2):
Determination of ABTS•+ scavenging capacity
The scavenging of the radical cation of 2,2′-azino-bis(3-ethylbenzothiazoline 6-sulfonic acid) (ABTS•+) by extracts was evaluated using the Re et al. [1999] method. This cation radical was formed after incubation of the ABTS (7 mM) with potassium persulfate (2.45 mM) at room temperature, under dark conditions, for 12 h. Once formed, it was diluted with ethanol until an absorbance value of 0.700±0.100 at 754 nm was obtained. The absorbance of ABTS•+ solution, reaction mixtures (Ae) and blank samples (Ab) was measured using UV/Vis Smartec Plus spectrophotometer (Bio-Rad). Reaction mixtures were obtained after reaction of 50 μL of extracts with 2.95 mL of ABTS•+ solution. A blank sample contained ethanol instead of extract. The percentage of ABTS•+ inhibition was calculated using the formula (3):
Fourier transform infrared spectroscopy analysis
The non-extruded and extrudate samples were dried for 24 h at 60°C and Fourier transform infrared spectroscopy (FTIR) analysis was carried out using a Spectrum 400 FTIR spectrometer (Perkin-Elmer, Waltham, Massachusetts, USA). The analysis conditions were marked by 40 scan cycles and by 2 cm−1 resolution being measured in the range of wavenumbers of 500–4,000 cm−1, using the transmittance mode.
Scanning electron microscopy analysis
The scanning electron microscope (SEM) images were only taken for the non-extruded tamarind shell and in extrudates that presented the best results of the chemical and physicochemical characterization, which corresponded to the treatment of tamarind shell with 32 g moisture/100 g at 100°C. Both samples were sieved with 60 mesh to obtain a uniform particle size of 250 μm, and were submitted to dehydration for 24 h at 60°C. Then, the samples were coated with copper and analyzed at 500× magnification using JMS-6400 scanning electron microscope with Bruker X FLASH 4010 SEM microanalysis detector (JEOL, Peabody, MA, USA).
Statistical analysis
All the chemical analyses were performed in triplicate and were later statistically processed with the JMP®6.0 software (Cary, NC, USA), using an analysis of variance (ANOVA) and a Tukey’s test in order to compare the treatments and observe whether differences were statistically significant at p<0.05. Finally, the correlations between the response variables were evaluated with the Pearson correlation coefficient (r).
RESULTS AND DISCUSSION
Chemical composition of tamarind shell
The chemical composition of the raw tamarind shell is shown in Table 1. Evidently, the total dietary fiber (TDF) was the major component of the shell and it consisted of IDF (56.80 g/100 g DM) and SDF (1.20 g/100 g DM). In turn, the IDF portion was formed mainly by lignin (31.57 g/100 g DM) and cellulose (16.21 g/100 g DM) while the SDF was formed by pectin (0.93 g/100 g DM). The TDF content quantified in the tamarind shell was higher to that reported in commercial products such as Metamucil®, Fibra Kania®, Fibra Tarasca®, Nopalinaza Plus®, and Fibra Xotzil®, which reportedly contribute 52.72 g/100 g, 50.57 g/100 g, 33.60 g/100 g, 33.32 g/100 g, and 21.90 g/100 g of TDF, respectively [Martínez-Flores et al., 2009]. Thereafter, the next-largest component in the tamarind shell was the nitrogen-free extract (35.24 g/100 g DM), followed by ash (3.60 g/100 g DM) and protein (2.43 g/100 g DM).
Table 1
Chemical composition of the tamarind shell.
| Component | Content (g/100 g DM) |
|---|---|
| Total dietary fiber (TDF) | 58.00±0.69 |
| Insoluble dietary fiber (IDF): | 56.80±0.53 |
| Lignin | 31.57±0.38 |
| Cellulose | 16.21±0.03 |
| Hemicellulose | 9.00±0.34 |
| Others | 0.02 |
| Soluble dietary fiber (SDF): | 1.20±0.35 |
| Pectin | 0.93±0.1 |
| Others | 0.27 |
| Ash | 3.60±0.02 |
| Protein | 2.43±0.19 |
| Fat | 0.73±0.12 |
| Free nitrogen extract1 | 35.24 |
Effect of the extrusion on the dietary fiber composition of tamarind shell
The contents of the TDF, IDF and SDF of both the extruded and non-extruded tamarind shells are shown in Table 2. The extruded samples exhibited increases, on average, by 9.3%, 6.9%, and 138.3% of the TDF, IDF, and SDF, respectively, in comparison with their respective levels in the non-extruded material. In a study done by Stojceska et al. [2010] using extrusion to obtain gluten-free products with fruit as a source of dietary fiber, extrusion significantly increased the total dietary fiber content in gluten-free products. This increase could be a result of the interaction between DF and phenolics (hydroxycinnamic acids, resveratrol, tannins, and flavonoids), which possibly formed insoluble complexes that were quantified as DF. According to Bader et al. [2019], extrusion modifies dietary fiber by applying high temperature, pressure, and shear force on food to break down glycosidic bond of insoluble polysaccharides and converts them into lower molecular weight soluble compounds. Arribas et al. [2017; 2019] mentioned that extrusion caused a considerable redistribution of insoluble dietary fiber to the soluble form and documented that increase in SDF could be attributable to the formation of other compounds, such as, polysaccharide-protein complex, which are measured as soluble fiber. Chen et al. [2018] indicated that the insoluble fiber of extruded powder may be converted into soluble oligosaccharide by extrusion. In our study, with moisture level of 32 g/100 g and temperatures of 90°C and 100°C, the greatest changes were recorded in the three DF portions of the tamarind shell (Table 2).
Table 2
Content of total dietary fiber (TDF), insoluble dietary fiber (IDF), soluble dietary fiber (SDF), as well as water absorption capacity (WAC) and oil absorption capacity (OAC) of non-extruded tamarind shell (control) and its extrudates.
In general, lignin and cellulose contents in the extruded samples were higher, on average, by 12.5% and 29.9%, respectively, while hemicellulose content was lower, on average, by 58.0% than in the tamarind shell (Table 3). In particular, lignin content increased compared to the non-extruded material in the treatments at 100°C where the initial tamarind shell moisture content was 32 g/100 g and at 100°C and 110°C with tamarind shell having the moisture content of 39 g/100 g of sample. Likewise, the cellulose content increased compared to the non-extruded material when a sample with moisture content of 32 g/100 g was used at each of the temperatures tested, as well as in the treatment of tamarind shell containing 39 g water/100 g treated at 90°C and 100°C. The hemicellulose content, regardless of the extrusion conditions, was lower in the extrudates compared to the non-extruded material.
Table 3
Content of lignin, cellulose, and hemicellulose in non-extruded tamarind shell (control) and its extrudates (g/100 g DM).
Effect of the extrusion on water and oil absorption capacities and glucose dialysis retardation index of tamarind shell
The results of OAC and WAC measurements of tamarind shell and its extrudates are shown in Table 2. The OAC of the control sample was 2.42 g oil/g DM and a tendency to increase OAC as a result of extrusion was observed (an average 16%), although the differences between values for non-extruded and extruded samples were not significant (p≥0.05). OAC has been attributed to the physical entrapment of oil [Abah et al., 2020], and such oil entrapment in dietary fibers is mainly related to the surface properties of the particles, the overall charge density, and the hydrophilic nature of the fiber [Elleuch et al., 2011]. A high OAC of food product is important because, according to the information documented by Kesselly et al. [2022], fats act as flavor retainers and improve mouthfeel of foods. Moreover, ingredients with high OAC can be used as emulsifiers in food items that are rich in fat. In addition, the ability of reduction of cholesterol levels in blood is linked with a high OAC of dietary fiber of extrudates, as documented by Khan et al. [2018]. The OAC depends mainly on the porosity of the material, since the oil is lodged in the said sites, as well as on the surface area and the hydrophobicity character found in chemical structure of IDF. The WAC of non-extruded tamarind shell was 1.85 g water/g DM, and for extrudates, an average decrease in WAC of 5.4% was noted; however, these changes were statistically insignificant (p≥0.05) (Table 2).
The glucose dialysis retardation index (GDRI) is a useful in vitro indicator of the effect of dietary fiber on gastrointestinal glucose absorption delay [Li et al., 2022]. In Figure 1, it was observed that the greatest retardation capacity in the diffusion of glucose in the extruded tamarind shells and the control was in the first 30 min. It should be noted that the highest GDRI was obtained both in tamarind shell with 32 g/100 g of moisture treated at 90°C, 100°C and 110°C (the values oscillated between 52.3–59.7%) and in control (54.2%). On average, tamarind shells processed with 32 g/100 g of moisture had a GDRI of 57.2% compared to a GDRI of 18.8% for tamarind shells containing 39 g/100 g. At 60 and 120 min, a strong effect of tamarind shells extruded with 32 g/100 g of moisture, regardless of the temperature used on the GDRI was still observed. The GDRI was gradually lower at the end of 240 min in all the treatments and the control.
Figure 1
Glucose dialysis retardation index over time of the non-extruded tamarind shell and its extrudates obtained from raw material with moisture contents of 32 g/100 g and 39 g/100 g at extrusion temperature of 90–110°C.
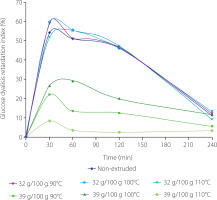
One of the salient physicochemical properties of a dietary fiber is its ability to retain organic molecules, such as glucose and lipids, in its structural matrix and form of a viscous gel [Martínez-Flores et al., 2009]. These viscous fibers can delay the gastric emptying of ingested food into the small intestine, providing a feeling of satiety, reducing postprandial blood glucose concentrations, and ensuring a beneficial effect on insulin sensitivity and weight control. As per available research, the extrusion process of a given raw material helps to increase the amount of its SDF, as persuasively documented by Bader et al. [2019]. Larrea et al. [2010], studying the effect of the fiber from the orange bagasse on the GDRI before and after the extrusion process, claimed that extruded samples presented a higher retardation rate compared to that of the non-extruded sample.
Effect of the extrusion on total phenolic content, total flavonoid content, and antioxidant capacity of tamarind shell
The total phenolic content of the tamarind shell was 19.86 mg GAE/g DM. In general, the extrudates exhibited significantly (p<0.05) lower total phenolic content (an average 40.3%) compared to the raw tamarind shell (Figure 2A). The lowest total phenolic content was 5.9 mg GAE/g DM, occurring in the tamarind shell with moisture content of 39 g/100 g extruded at 100°C. In turn, the total flavonoid content of the unprocessed tamarind shell was 13.54 mg RE/g DM. After the extrusion, a reduction in total flavonoid content of 7.16% was observed in the tamarind shell with a moisture content of 32 g/100 g treated at 100°C (Figure 2B). This sample had the highest total flavonoid content between extrudates. An average reduction in total flavonoid content in the extrudates compared to the non-extruded sample was 18.4%. The phenolic content was reduced in the extruded tamarind shell, probably due to its instability of phenolics in the conditions of process. The change of the chemical structure of phenolic compounds was attributed to the combined effect of moisture and extrusion temperature. In this regard, Sharma et al. [2012] mentioned that the use of high moisture was more destructive for these compounds. The synergistic effect of moisture and temperature above 80°C mainly generates the decarboxylation [Brennan et al., 2011], malonic conjugation, and de-esterification of phenolics [Mahungu et al., 1999].
Figure 2
Total phenolic content (A) and total flavonoid content (B) of the non-extruded tamarind shell and its extrudates obtained from raw material with moisture contents of 32 g/100 g and 39 g/100 g at extrusion temperature of 90–110°C. Different letters above bars indicate that the treatments are significantly different (p<0.05). GAE, gallic acid equivalents; RE, rutin equivalents; DM, dry matter.
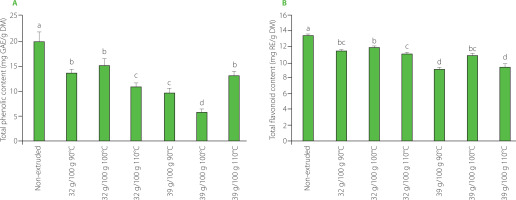
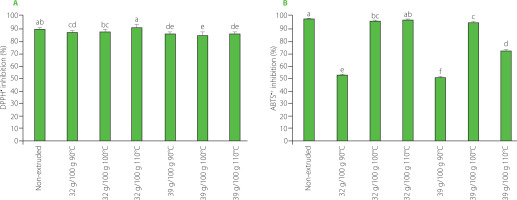
Figure 3A and 3B depict the results of measurements of the antioxidant capacity of non-extruded tamarind shell and its extrudates in ABTS and DPPH assays. There was an average decrease in the scavenging capacity of extrudates against both radicals compared to raw tamarind shell (by 2.8% in DPPH and 19.6% in ABTS assay), but, for both radicals, the scavenging capacity of tamarind shell with a moisture level of 32 g/100 g treated at 100°C and 110°C was similar to that for the non-extruded sample, indicating the maintenance of their antioxidant capacity.
Figure 3
DPPH• (A) and ABTS•+(B) scavenging capacity of the non-extruded tamarind shell and its extrudates obtained from raw material with moisture contents of 32 g/100 g and 39 g/100 g at extrusion temperature of 90–110°C. Different letters above bars indicate that the treatments are significantly different (p<0.05).
Correlation analysis
The TDF content showed a significant (p<0.001), positive, correlation with the IDF and with lignin contents (r=0.978 and r=0.825, respectively) (Table 4). Likewise, lignin correlated significantly (p<0.001) with IDF (r=0.881). The hemicellulose content also exhibited affinity for total phenolic content (r=0.732, p<0.001) and total flavonoid content (r=0.586, p<0.01). Total flavonoid content had a significant (p<0.01) affinity towards the ABTS•+ scavenging capacity (r=0.646) and DPPH• scavenging capacity (r=0.561), while total phenolic content was significantly (p<0.01) correlated with the DPPH• scavenging capacity (r=0.552).
Table 4
Coefficients of Pearson correlations of response variables for the non-extruded tamarind shell and its extrudates.
| TDF | IDF | SDF | Total phenolic content | Total flavonoid content | DPPH assay | ABTS assay | Lignin | Celluolose | |
|---|---|---|---|---|---|---|---|---|---|
| IDF | 0.978*** | ||||||||
| SDF | 0.639** | 0.476 | |||||||
| Total phenolic content | 0.223 | 0.222 | 0.013 | ||||||
| Total flavonoid content | 0.120 | 0.085 | 0.162 | 0.490 | |||||
| DPPH assay | 0.011 | −0.100 | 0.285 | 0.552** | 0.561** | ||||
| ABTS assay | 0.139 | 0.151 | 0.070 | 0.148 | 0.646** | 0.452 | |||
| Lognin | 0.825*** | 0.881*** | 0.313 | 0.079 | 0.053 | −0.308 | 0.264 | ||
| Cellulose | 0.323 | 0.224 | 0.469 | −0.090 | −0.493 | 0.027 | −0.526 | −0.050 | |
| Hemicellulose | −0.256 | −0.226 | −0.366 | 0.732*** | 0.586** | 0.501* | 0.191 | −0.277 | −0.498 |
FTIR spectra and SEM micrographs of tamarind shell and its extrudates
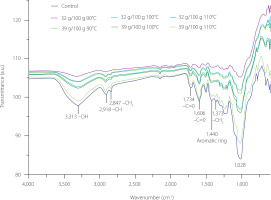
The FTIR spectra obtained from the extruded samples were similar among themselves as well as with respect to the non-extruded sample (Figure 4). The signals that corresponded to different functional groups are present in the spectra. A broad band at a wavenumber of 3,313 cm−1 (Figure 4) would indicate the stretching of the hydroxyl groups (–OH) of cellulose, lignin, and polyphenols [Anirudhan et al., 2008], in addition to the stretching vibration generated by the –OH of the carboxyl groups (–COOH) of pectin and hemicellulose molecules [Arroyo et al., 2008; Sivam et al., 2011]. Pectin is a linear polysaccharide comprising several d-galacturonic acid molecules connected by α-1,4 glycosidic bonds [Liu et al., 2021]. The stretching vibration of the hydroxyl groups in pectin is generally attributed to the presence of d-galacturonic acids [Arroyo et al., 2008]. On the other hand, hemicelluloses, having a heterogeneous chemical structure, exhibit the said stretching vibrations in their different functional groups, corresponding to those of the monosaccharides such as arabinose, xylose, glucose, mannose, galactose, and uronic acids [Arroyo et al., 2008]. In this study, the characteristic signal of hydroxyl groups was also observed at a wavenumber of 1,232 cm−1, corresponding with lignin, and at wavenumbers of 2,916 cm−1, 2,847 cm−1, and 1,373 cm−1, associated with the elongation vibrations of the carbon-hydrogen of the alkane group (–CH) of cellulose, hemicellulose, and lignin, respectively [Arroyo et al., 2008]. The bands at 1,734 cm−1 and 1,605 cm−1 of the carbonyl group (–C=O) were associated with the aldehydes and ketones of simple and complex carbohydrates and the esterified carboxylic acids from pectin, hemicellulose, and lignin [Arroyo et al., 2008]. Sivam et al. [2011] related the 1,734 cm−1 wavenumber with the presence of methylated pectin. The C–O asymmetric stretching of the hydroxyl groups of phenolics was observed at 1,028 cm−1 wavenumber. The band at 2,916 cm−1 wavenumber is characteristic of catechin, in addition to 2,847 cm−1 being associated with gallic acid and, 3313 cm−1 being related to tannic acid.
Figure 4
Fourier transform infrared spectra of the non-extruded tamarind shell (control) and its extrudates obtained from raw material with moisture contents of 32 g/100 g and 39 g/100 g at extrusion temperature of 90–110°C.
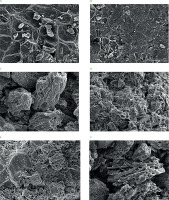
The micrographs obtained for the tamarind shell under different conditions are shown in Figure 5. The morphology of the inner part of the tamarind shell without grinding (Figure 5A) showed a heterogeneous surface with smooth and rough parts, while the external part (Figure 5B) displayed a much rougher surface resembling that of a honeycomb. Similar morphology of tamarind shell was also reported by Sivasankar et al. [2012]. Figure 5C presents the micrograph of ground non-extruded tamarind shell. Small, isolated fragments with a porous structure were visible along with striations due to the presence of cellulose microfibrils. This characteristic porosity would give DF the ability to trap oil molecules, as was documented by Zhuang et al. [2019]. Further, the micrographs presented in Figures 5D–E show the morphology of the ground and extruded shell, implying a more interconnected (homogenized) structure where the isolated shell fragments were not observed. In addition, the extruded material had small pores on its surface. After the extrusion process, the cellulose present in the extruded tamarind shell (Figure 5F) presented a well-defined cylindrical cell structure, which also coincided with the report documented by Maheswari et al. [2008].
Figure 5
Scanning electron micrographs of internal (A) and external (B) part of the non-extruded and non-ground tamarind shells, non-extruded ground tamarind shell (C), extruded tamarind shells at 100°C and initial moisture contents of 32 g/100 g (D) and 39 g/100 g (E), and cellulose microfibrils in the extrudate obtained at 110°C and initial moisture content of 39 g/100 g (F). Magnification 500×.
CONCLUSION
The temperature of the extrusion and moisture content of the tamarind shell subjected to extrusion affected changes in the chemical composition of the extrudates, mainly in terms of their soluble dietary fiber content and improved some of the raw material’s physiochemical properties, such as OAC and GDRI, suggesting the potential benefits of using the extruded tamarind shell. The treatment of shell with a moisture content of 32 g/100 g at 100°C was particularly beneficial for improving these parameters. Although the general aim of extrusion of tamarind shell was to reduce the content of phenolic compounds, extrusion of shells with a moisture content of 32 g/100 g at 100°C and 110°C allowed to maintain antioxidant capacity, and the extrudates had DPPH• and ABTS•+ scavenging activity at the level of the starting material. In light of the above findings, this item, hitherto considered by the food industry as waste material, could be used in the future as a raw material in the production of functional foods.

