INTRODUCTION
Emulsion-type meat products come into existence through the emulsification of muscle proteins, lipids, water, and assorted non-meat additives. The emulsification mechanism is explained by two hypotheses. The initial theory, termed oil-in-water, involves the formation of a myofibrillar protein film around fat particles to prevent the accumulation of fat globules. The second theory, known as the physical entrapment theory, explains that fat globules are embedded in a protein network and that the stabilization of fat particles occurs through the myofibrillar protein gel network [Gordon et al., 1992]. To prepare meat emulsion, myofibrillar proteins, which can be soluble in water with salt and act as emulsifiers, are first extracted from lean meat through the chopping process, then animal fat is also finely chopped and added to the meat batter. The chopping process is used to produce a meat emulsion from protein extracted from lean meat, fat and water [Gordon et al., 1992; Zhao et al., 2019]. The fat particles stabilized in this matrix affect the sensory properties, technological characteristics (water-holding capacity, cooking yields) and textural attributes of the meat [Kumar et al., 2016; Zhao et al., 2019]. However, concerns about the adverse health effects of excessive fat consumption have prompted extensive research around the world into the production of low-fat and healthier meat products [Chappalwar et al., 2020; Choi et al., 2014; Kumar et al., 2016].
One of the ways to produce meat products that are both healthy and functional is to use different plant materials rich in dietary fiber as a functional ingredient [Das et al., 2020]. Consuming sufficient dietary fiber has been reported to reduce the risk of developing some chronic diseases, such as colon cancer, obesity and cardiovascular disease, by lowering cholesterol and triglyceride levels [WHO/FAO, 2003]. Various dietary fibers, with different non-starch polysaccharide compositions (pectins, hemicelluloses, lignins, etc.), show not only positive effects on health, but also have different technological characteristics, including gelling properties, that can improve the emulsification, and can influence the sensory rheological, and the desirable textural properties of processed meat products [Das et al., 2020; Unal et al., 2022].
The banana is widely recognized as one of the most commonly consumed tropical fruits, known for its high carbohydrate, dietary fiber, mineral, and vitamin content. It is also rich in several compounds with antioxidative activity, such as carotenoids, phenolics, ascorbic acid, and tocopherols [Singh et al., 2016]. The banana peel, a major by-product of the food industry, accounts for approximately 30% of the fruit’s total weight [Schieber et al., 2001]. Many studies have highlighted the potential of banana peels, which are a burden on the environment, to be used in the food industry, especially in the production of bakery products and pasta [Gomes et al., 2022; Puraikalan, 2018; Salama et al., 2019], although recently also a meat product (chicken sausage) with the addition of banana peel powder has been formulated [Zaini et al., 2020]. They are of interest due to a high dietary fiber content (40–50 g/100 g dry matter, dm), mainly the insoluble fraction, which accounts for 75% of the total dietary fiber [Emaga et al., 2007]. Another component of banana peels, important in terms of their use as a food ingredient, are phenolic compounds, including dominant proanthocyanidins with a high degree of polymerization, procyanidin dimers, flavan-3-ols and flavonol glycosides, which are responsible for their higher antioxidative potential compared to banana pulp [Rebello et al., 2014; Someya et al., 2002]. Banana peels are also a source of other bioactive compounds like carotenoids and biogenic amines [Pereira & Maraschin, 2015]. Additionally, they contain essential minerals like potassium, protein (8–11 g/100 g dm), polyunsaturated fatty acids, and essential amino acids [Emaga et al., 2007]. Despite attempts to use this cheap and easily accessible material in food production, there is a lack of research on the use of banana peel albedo as a functional ingredient in emulsion-type meat products. Therefore, this study aimed to explore the effects of banana peel albedo powder (BPAP) on the technological and microstructural properties of beef meat emulsions.
MATERIALS AND METHODS
Banana peel albedo powder preparation
Banana fruits (approximately 5 kg) were collected from the Mediterranean Region of Türkiye (Antalya). The banana albedo used in this study was obtained by carefully removing the white inner layer (albedo) from the banana peel surrounding the fruit. The albedo was then air-dried at room temperature (at approximately 25°C) for 48 h. After drying, the albedo was pulverized using a grinder and sifted through a 60-mesh screen to obtain a fine powder. The resulting banana peel albedo powder (BPAP) was stored in airtight packaging at 15±2°C in cold storage until further analysis.
Preparation of beef meat emulsions
Fresh raw beef, with the moisture content of 72.01±0.25 g/100 g, protein content of 19.21±0.08 g/100 g, fat content of 8.29±0.47 g/100 g, and pH of 5.89±0.01, was purchased from a butcher in Konya, Türkiye. To prepare the beef meat emulsions, the methodology detailed in our previous study [Unal et al., 2022] was used, with slight modifications such as mixing time and oil temperature. To this end, 25 g of raw ground beef without BPAP (0.0%, control) or with BPAP at different levels (0.5%, 1.0%, and 1.5% of the weight of raw ground beef, w/w) were mixed with 100 mL of an NaCl (2.5%) + K2HPO4 (0.5%) solution and blended for 120 s in a Waring Commercial 8011 blender (Stamford, CT, USA), resulting in four separate slurries. In the next step, the slurries (12.5 g) were blended with 37.5 mL of an NaCl (2.5%) + K2HPO4 (0.5%) solution and 50 mL of corn oil (9°C). An oil was added at a stable rate using a system described by Ockerman [1985] with a cold water-jacketed burette (4°C). The dispersion was carried out at 13,000 rpm for 20 s until a uniform mixture was obtained, ensuring consistent texture. The BPAP contents in the final emulsions were 0.0%, 0.013%, 0.025%, and 0.038% (w/v), respectively.
The experiment was conducted with three replications, e.g., emulsions for each formulation were prepared separately in triplicate, and all analyses were conducted on the three independent emulsions for each formulation. The prepared emulsions were stored under controlled conditions until further analysis.
Determination of dietary fiber content and mineral profile of banana peel albedo powder
The dietary fiber content (soluble and insoluble) of BPAP was determined according to the AACC International method [AACC, 1999]. The soluble and insoluble dietary fiber contents were expressed in g/100 g BPAP.
For the determination of mineral content, 1 g of dry BPAP was subjected to wet ashing using sulfuric acid and hydrogen peroxide, as described by Babiker et al. [2021]. The mineral content in the resulting ash was analyzed using inductively coupled plasma atomic emission spectroscopy (ICP-AES). Varian-Vista ICP-AES instrument (Varian, Inc., Mulgrave, Victoria Australia) was used, which operated under the following conditions: radio frequency plasma power was maintained at 0.7–1.5 kW (1.2–1.3 kW for axial); plasma Ar flow rate was 10.5–15 L/min (radial) and 15 L/min (axial); auxiliary Ar flow rate was kept at 1.5 L/min; the viewing height was 5–12 mm; the copy and reading time was set at 1–5 s (max. 60 s), and the copy time was 3 s (max. 100 s) [Babiker et al., 2021]. Quantification of minerals was carried out using data on similar minerals obtained by analysis of certified reference samples from the National Institute of Standards and Technology (NIST; Gaithersburg, MD, USA) [Skujins, 1998]. The mineral contents were expressed as mg per 100 g of BPAP.
Determination of total phenolic content, total flavonoid content and antioxi dative activity of banana peel albedo powder
The procedure suggested by Cetin‐Babaoglu et al. [2024] was modified to acquire the extract from the BPAP to determine the total phenolic content (TPC), total flavonoid content (TFC), 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity, and ferric reducing antioxidant power (FRAP). Five grams of ground BPAP were suspended in 100 mL of 70% methanol (v/v) for this purpose, and incubated for 1 h at 25°C in a shaking water bath (250 rpm). Following incubation, the supernatant was separated using centrifugation at 13,400×g for 10 min.
To determine the TPC, 0.1 mL of the extract, 0.4 mL of distilled water, and 2.5 mL of a 10% Folin-Ciocalteu’s reagent (v/v) were placed in a tube and kept in the dark for 2 min [Škerget et al., 2005]. The sample tube was then filled with 2 mL of a 7.5% Na2CO3 (w/v) solution and incubated at 50°C for 5 min. The absorbance of the reaction mixture was measured at 760 nm using a UV–Vis spectrophotometer (UV-160 A, Shimadzu, Tokyo, Japan). The TPC of the BPAP was ascertained using a gallic acid standard curve, and results were expressed as mg gallic acid equivalent (GAE)/g.
To determine the TFC, 150 μL of 5% NaNO3 (w/v), 2 mL of distilled water, and 0.5 mL of the extract were put in a tube and left in the dark for 6 min [Eyiz et al., 2020]. The sample tube was then filled with 200 μL of distilled water, 2 mL of 1 M NaOH, and 150 μL of a 10% AlCl3 (w/v) solution, and it was left to stand for 15 min. The absorbance of the mixture was measured at 510 nm using a spectrophotometer (UV-160 A, Shimadzu). The TFC of the BPAP was calculated using a catechin standard curve, and results were expressed as mg catechin equivalent (CE)/g.
The DPPH radical scavenging activity of the BPAP was determined using the method described by Brand-Williams et al. [1995]. Briefly, the BPAP extract (50 µL) was added to 2,950 µL of a 100 µM methanolic DPPH radical solution, and the reaction mixture was incubated in the dark for 30 min. The absorbance was measured at 517 nm using a spectrophotometer (UV-160 A, Shimadzu). The DPPH radical scavenging activity of BPAP was expressed as Trolox equivalents (µmol Trolox/g), calculated using a calibration curve prepared with standard Trolox solutions.
Then, 10 mL of a 2,4,6-tri(2-pyridyl)-s-triazine solution (10 mM in a 40 mM hydrochloric acid solution), 100 mL of sodium acetate buffer (300 mM, pH 3.6), and 10 mL of a ferric chloride hexahydrate solution (20 mM) were combined to achieve the FRAP reagent [Benzie & Strain, 1996]. After that, 225 μL of distilled water, 2.25 mL of the FRAP reagent, and 75 μL of the extract were vortexed and allowed to stand at room temperature for 30 min. The samples’ absorbance was measured at 593 nm. Iron(II) sulfate heptahydrate was used to plot the standard curve, and the findings were reported as mg Fe2+/kg BPAP.
pH measurement of beef meat emulsions
The pH of the beef meat emulsions was measured following the procedure outlined by Zorba et al. [1993]. A pH meter (WTW series pH 720, Weilheim, Germany) was used to assess the pH values. To this end, 10 g of each emulsion sample was homogenized with 100 mL of distilled water, and the pH was directly measured after the calibration of the instrument with standard buffer solutions at pH 4.0 and 7.0. The readings were taken immediately after calibration to ensure accuracy.
Measurements of color coordinates of beef meat emulsions
The color coordinates of the raw and cooked (cooking conditions as for determining cooking loss) beef meat emulsions were measured in the CIELab space using a Minolta chromameter CR-400 (Konica Minolta, Osaka, Japan), in accordance with the method described by Hunt et al. [1991]. The instrument had a measurement area diameter of 8 mm and a range of values from 0 to 100 for L* (lightness), ±60 for a* (red/green), and ±60 for b* (yellow/blue). The measurements were taken under D65 illuminant with a 2° observer angle, ensuring high accuracy for surface color analysis. Each sample was measured five times to ensure consistency, and the average values were recorded. The results reflect the surface color characteristics of the emulsions, providing comprehensive data on their visual appearance.
Determination of beef meat emulsion cooking loss and water holding capacity
The cooking loss (CL) of the beef meat emulsions was determined following the method described by Mejia et al. [2018]. For each formulation, exactly 30 g of the emulsion were portioned into 50 mL polypropylene tubes. The samples were then subjected to thermal treatment in a water bath (Nüve, Ankara, Türkiye), beginning at 50°C and gradually increasing the water temperature until the internal temperature of each sample reached 72°C, which was monitored using a thermometer probe. Following heating, the tubes were cooled in ice water for 5 min, inverted, and stored at 4°C for 14 h to collect the released exudate. The difference in weight before and after cooking was used to determine CL, expressed as a percentage based on the initial sample mass.
The water holding capacity (WHC) of the beef meat emulsions was evaluated using a modified version of the method by Hughes et al. [1997]. Ten grams of uncooked emulsion batter were placed into glass jars and heated in a 90°C water bath for 10 min. After cooling to room temperature, the samples were wrapped in cotton cheesecloth and centrifuged at 7,245×g for 15 min. WHC was determined by measuring the amount of water retained after heating and centrifugation, relative to the total moisture content of the sample. Results were expressed as a percentage of retained water.
Analysis of emulsifying properties of beef meat emulsions
The emulsifying properties, including emulsifying capacity (EC) and emulsion stability (ES), were determined according to the method of Ockerman [1985]. The endpoint for emulsification was identified using the procedure described by Webb et al. [1970]. To measure EC, the corn oil was added to the beef meat emulsion from a burette, and the electrical conductivity was simultaneously monitored by an ohmmeter (YX-360TR N Multitester Fuse and Diode Protection, Sunwa, Tokyo, Japan) with an electric potential (mV) recorder (Labsco Laboratory Supply, Louisville, KY, USA). At the emulsification endpoint, the conductivity suddenly decreased, a sudden reduction in resistance was observed in the ohmmeter reading. The oil addition was stopped, and the total volume of emulsified oil (added to beef meat emulsion) was recorded from the burette. EC was calculated as mL of oil retained per g of protein. The protein content of the beef meat was determined by the Kjeldahl method [AACC, 1999].
To determine ES, an emulsion with corn oil was formed as described above. However, the emulsification was stopped when 110 mL of oil was used. Then, 20 g of the emulsion were weighed into test tubes and heated in a water bath (Nüve) at 80°C until the core temperature reached 72°C. The tubes were centrifuged and drained into a volumetric cylinder for 10 h to collect the unbound oil and water. ES was calculated according to Equation (1):
where: SO is the amount of separated oil released by the emulsion and SW is the amount of separated water released by the emulsion [Ockerman, 1985].Determination of apparent viscosity, flow behavior index and consistency index of beef meat emulsions
Apparent viscosity (AV) of the newly formed beef meat emulsions (approximately 25 g), kept at a constant temperature of 20oC using a thermostatically controlled water bath, was measured at four different rotational speeds (10, 20, 50, and 100 rpm) using a rotational viscometer (Brookfield, DV2TRVTJ0, Middleborough, MA, USA). Then, the flow curves, e.g., apparent viscosity vs. shear rate, were plotted. Using linear regression analysis, the data were fitted to the power law model presented in Equation (2) to determine the flow behavior index (n), consistency index (k), and determination coefficients (r2) [Barnes et al., 1989]:
where: ηa is the apparent viscosity (mPa×s), k is the consistency index (Pa×sn),
Texture profile analysis of beef meat emulsions
Texture profile analysis (TPA) of the beef meat emulsions was performed using a Stable Micro Systems Texture Analyser (model: TA.XT Plus, Godalming, UK). For each formulation, exactly 30 g of the emulsion were portioned into 50 mL Falcon tubes, which were then cooked in a water bath (Nüve) at 80°C until the core temperature reached 72°C. This process took approximately 20 min. After cooling to room temperature, the samples were removed from the tubes and cut into cylindrical shapes with a diameter equal to the tube’s internal diameter (28 mm) and a height of 15 mm for TPA measurements. Seven representative samples were randomly selected from each analyzed emulsion to ensure adequate representation of the overall texture profile. A cylindrical plate with a diameter of 36 mm and a 50 kg load cell were used. Each sample was compressed twice, with a 0.1 s delay between the descents, at a distance of 5 mm. The following test parameters were applied: pre-test speed of 1 mm/s, test speed of 5 mm/s, post-test speed of 5 mm/s, and 50% compression. TPA was conducted at room temperature (21°C). The following textural parameters were determined: hardness (N), adhesiveness (N×s), springiness (mm), cohesiveness, and gumminess (N). Hardness was determined by the maximum force required to compress the sample, cohesiveness was calculated as the ratio of the positive force area during the second compression to that during the first compression, and springiness was determined as the distance the sample recovered after the first compression. Adhesiveness was calculated as the negative force area during the first compression, while gumminess was defined as the product of hardness and cohesiveness [Herrero et al., 2007].
Microstructural analysis of beef meat emulsions
Microstructural analysis, including the acquisition of confocal laser scanning microscope (CLSM) images, was conducted using a Nikon A1R1 instrument (Nikon, Tokyo, Japan), equipped with a helium/neon laser at the fluorescence excitation of 520–530 nm in accordance with the method specified by Zhu et al. [2018]. The resolution of the images was 1,024×1,024 pixels, and 10 images were captured for each sample.
Statistical analysis
The differences were considered significant at p<0.05. Results related to the BPAP itself, such as dietary fiber content, mineral profile, total phenolic content, total flavonoid content, and antioxidative activity, were expressed as mean ± standard deviation (SD). In contrast, results for the emulsions (including both BPAP-added and control samples) were presented as mean ± standard error (SE). Data were analyzed using one-way analysis of variance (ANOVA) to determine significant differences among the beef meat emulsions with different contents of BPAP. The statistical analysis was performed using Minitab software, version 16.0 (Minitab, LLC, State College, PA, USA). Post-hoc comparisons were made using Tukey’s honestly significant difference (HSD) test. The assumption of normality and homogeneity of variances was checked prior to conducting ANOVA.
RESULTS AND DISCUSSION
Dietary fiber and mineral contents of banana peel albedo powder
The dietary fiber content as insoluble and soluble fractions of banana peel albedo powder is shown in Table 1. The content of insoluble dietary fiber of BPAP was found as 25.19 g/100 g. Moreover, its soluble dietary fiber was determined as 6.20 g/100 g. Emaga et al. [2007] analyzed the dietary fiber content in banana and plantain peels from different varieties and fruits of different ripeness and reported higher soluble and insoluble dietary fiber content in dessert banana peels (36.3–42.9% and 6.8–13.7%, respectively) compared to the content of these fractions in powdered banana peel albedo found in our study. In another study, the soluble and insoluble dietary fiber content of banana peels was found as 9.60 and 32.00%, respectively [Salama et al., 2019]. In turn, Zaini et al. [2020] prepared banana peel powder with total dietary fiber content of 44.03%.
The mineral content of banana peel albedo powder is presented in Table 2. Contents of individual minerals were found as: 3.85 mg/100 g for calcium, 0.27 mg/100 g for iron, 90.83 mg/100 g for potassium, 2.85 mg/100 g for magnesium, 1.11 mg/100 g for sodium, 9.47 mg/100 g for phosphorus, and 0.1 mg/100 g for zinc. Romelle et al. [2016] found the Ca, Zn, Fe, and Mn contents of banana peel as 19.86, 1.72, 15.15, and 9.05 mg/100 g dry peel, respectively. It is thought that various factors like soil condition, state of fruit ripeness, plant variety, irrigation regime, soil type, and which part of the fruit is used can cause differences in the mineral contents of a given type fruit sourced from different regions [Bartual et al., 2022; Emaga et al., 2007]. However, it was concluded that banana peel albedo is a source of dietary fiber and some minerals, so when used in product formulations, it will increase the dietary fiber and mineral content of the final product.
Total phenolic content, total flavonoid content and antioxidative activity of banana peel albedo powder
The TPC and TFC of BPAP were found as 9.16 mg GAE/g and 2.67 mg CE/g, respectively (Table 1). The DPPH radical scavenging activity of BPAP was determined to be 97.90 µmol Trolox/g, and the FRAP was 45.40 µmol Fe+2/g.
BPAP was a rich source of phenolic compounds compared to by-products from other tropical fruits, e.g., Can-Cauich et al. [2017] found a TPC of 210 mg GAE/100 g in mamey sapote peel, Selani et al. [2016] reported TPC in guava and passion fruit by-products to be 254.7 and 175.6 mg GAE/100 g, respectively, and the TPC determined by Lopez-Martinez et al. [2023] for guava, mamey sapote, and passion fruit peels ranged from 185.2 to 365.7 mg GAE/100 g. Regarding banana fruit, Someya et al. [2002] showed that banana peel contained four times more total phenolics than pulp.
In turn, Rebello et al. [2014] reported that TPC of banana peel was 29.2 mg GAE/g. According to Salama et al. [2019], the banana peel had TPC of 71.685 mg GAE/g. These values were higher than the TPC shown in Table 1; however, the whole banana peel was analyzed in the cited studies. To the best of our knowledge, the TPC of only the albedo part of the peel has not been determined so far. In addition to TPC, also the TFC found in our study for the BPAP was lower than that reported for banana peel expressed as rutin equivalents (3.354 mg RE/g) [Salama et al., 2019].
Table 1
Mineral composition, dietary fiber content, total phenolic content (TPC), total flavonoid content (TFC) content, and antioxidative activity of banana peel albedo powder (BPAP).
Cooking loss and water holding capacity of beef meat emulsions
The results shown in Table 2 indicate that the incorporation of BPAP into the beef meat emulsion affected its CL and WHC. The WHC tended to increase, while the CL tended to decrease with an increasing level of BPAP in the beef meat emulsion; however, significant differences (p<0.05) were found only between the emulsion with the highest level of BPAP (1.5% of raw beef meat, w/w, corresponding to 0.038% of emulsion, w/v) and the control. These changes could be due to the present of dietary fiber in banana peel. Overall, these findings were in agreement with our previous research, wherein the use of pumpkin powder decreased the cooking loss of beef by 17.38%–21.99% [Unal et al., 2022].
Table 2
Cooking loss and water holding capacity of beef meat emulsions with banana peel albedo powder (BPAP) added at different levels.
| BPAP level (% raw beef, w/w)* | Cooking loss (%) | Water holding capacity (%) |
|---|---|---|
| 0.0 (Control) | 10.47±2.61a | 15.71±0.41b |
| 0.5 | 6.10±0.14ab | 17.96±0.77ab |
| 1.0 | 5.24±0.18ab | 18.31±0.56ab |
| 1.5 | 4.76±0.93b | 18.95±1.34a |
pH value of raw beef meat emulsions
The pH values of raw emulsions were 7.25–7.26 (Table 3). BPAP use in emulsion formula had no significant (p≥0.05) effect on pH. There are conflicting reports regarding the pH values of meat products and the addition of different sources of fibers. Fernández-Ginés et al. [2003] reported that the pH values of bologna sausages did not change significantly as a result of the addition of citrus fiber. In contrast, Chappalwar et al. [2020] reported that the incorporation of banana peel flour in chicken patties caused a decrease in pH. Sarıçoban et al. [2008] also found a gradual decrease in pH of model meat emulsions with the increase in the level of lemon albedo added to them.
Table 3
pH and color parameters of raw beef meat emulsions with banana peel albedo powder (BPAP) added at different levels.
[i] *The final BPAP content in the emulsions was 0.013%, 0.025%, and 0.038% (w/v) for BPAP added at 0.5%, 1.0%, and 1.5% of raw beef (w/w), respectively. Results are shown as mean ± standard error. Values with different letters (a–b) within the column differ significantly (p<0.05). L*, lightness (0 – black, 100 – white); a*, redness-greenness (+a* – red, –a* – green); b*, yellowness-blueness (+b* – yellow, –b* – blue).
Color parameters of raw and cooked beef meat emulsions
Color parameters of raw and cooked emulsions are shown in Table 3 and 4, respectively. The incorporation of BPAP did not affect the lightness and yellowness of raw emulsions (p≥0.05). The L* values were in the range of 85.18–85.57 and were higher than those measured for the cooked beef emulsions (71.46–76.13). The cooked emulsion with BPAP added at a level of 1.5% of raw beef meat (w/w) was characterized by significantly (p<0.05) lower lightness compared to the control. A similar effect of reducing the lightness of corn pasta and extrudate after replacing 5% of flour with banana peel powder was observed by Puraikalan [2018].
Table 4
Color parameters of cooked beef meat emulsions with banana peel albedo powder (BPAP) added at different levels.
| BPAP level (% raw beef, w/w)* | L* | a* | b* |
|---|---|---|---|
| 0.0 (Control) | 76.13±1.15a | −2.44±0.15a | 15.47±0.71a |
| 0.5 | 76.08±0.47a | −2.28±0.02a | 14.56±0.22a |
| 1.0 | 74.38±0.30a | −2.28±0.03a | 14.58±0.53a |
| 1.5 | 71.46±0.11b | −2.22±0.05a | 15.41±0.48a |
* The final BPAP content in the emulsions was 0.013%, 0.025%, and 0.038% (w/v) for BPAP added at 0.5%, 1.0%, and 1.5% of raw beef (w/w), respectively. Results are shown as mean ± standard error. Values with different letters (a–b) within the column differ significantly (p<0.05). L*, lightness (0 – black, 100 – white); a*, redness-greenness (+a* – red, –a* – green); b*, yellowness-blueness (+b* – yellow, –b* – blue).
Regarding the a* value, the addition of BPAP caused significant (p<0.05) changes only in raw emulsions, for which the a* values ranged from −1.00 to −0.41 (Table 3). The BPAP addition at the level of 0.5% of raw beef meat (w/w) resulted in a higher (p<0.05) a* value in raw sample compared to the control emulsion, whereas no significant (p≥0.05) differences in a* values were observed among the emulsions with BPAP content. Additionally, there was no significant (p≥0.05) effect of BPAP incorporation on b* values of both raw and cooked emulsions.
Huang & Bohrer [2020] reported that the addition of commercial banana flour at 1%, 2%, and 4% levels increased a* values and decreased b* values in beef emulsions after cooking. Conversely, Kumar et al. [2013] observed that replacing 3% and 4% of lean meat with green banana flour in cooked chicken nuggets did not affect a* values, while a 5% substitution significantly reduced them. These findings indicate that banana flours may enhance the red color of cooked and cured meat products, although the effects appear to vary depending on the flour origin.
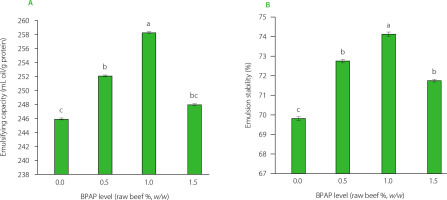
Beef meat emulsion capacity and stability
The ES and EC of beef meat emulsions with different contents of BPAP are shown in Figure 1. The control sample had the lowest EC and ES. Adding BPAP to the emulsions at the levels up to 1.0% of raw beef meat, w/w (up to 0.025% of emulsion, w/v), gradually increased their EC and ES. A further increase of the additive content caused a decrease of both parameters. Similar results were obtained by dos Santos Alves et al. [2016], who investigated the effect of pork skin and green banana flour blends as fat substitutes in bologna-type sausages. They reported greater emulsion stability when up to 60% fat substitute was included to the product. In contrast to our results, Chappalwar et al. [2020] found that banana peel powder did not affect the ES of chicken patties.
Figure 1
Emulsifying capacity (EC) (A) and emulsion stability (ES) (B) of beef meat emulsions with banana peel albedo powder (BPAP) added at 0.0%, 0.5%, 1.0%, and 1.5% based on the weight of the raw beef used. The actual contents of BPAP in the emulsions were 0.0%, 0.025%, 0.038%, and 0.075% (w/v), respectively.
Apparent viscosity of cooked beef meat emulsions
The flow curves of cooked beef meat emulsions are shown in Figure 2. In addition, the parameters of power law model, including flow behavior index and consistency index, determined for all emulsions are presented in Table 5. The consistency index was in the range of 64,206 and 92,667 mPa×sn. The emulsion with BPAP added at the level of 1.0% of raw beef meat, w/w (0.038% of the emulsion, w/v) had the highest consistency index of 92,667 mPa×sn, while the lowest flow behavior index of 0.137. Therefore, the flow behavior index showed completely reverse trend with consistency index. Similar findings were reported by Sarıçoban et al. [2010], who investigated rheological properties of beef emulsions with the addition of sunflower head pith.
Table 5
Consistency index (k) and flow behavior index (n) of beef meat emulsions with banana peel albedo powder (BPAP) added at different levels.
| BPAP level (% raw beef, w/w)* | n | k (mPa×sn) | r2 |
|---|---|---|---|
| 0.0 (Control) | 0.177±0.00a | 64,206±979c | 0.999 |
| 0.5 | 0.144±0.02b | 88,823±4,233b | 0.990 |
| 1.0 | 0.137±0.01c | 92,667±1,578a | 0.998 |
| 1.5 | 0.142±0.01b | 66,068±537c | 0.996 |
* The final BPAP content in the emulsions was 0.013%, 0.025%, and 0.038% (w/v) for BPAP added at 0.5%, 1.0%, and 1.5% of raw beef (w/w), respectively. Results are shown as mean ± standard deviations. Values with different letters (a–c) within the column differ significantly (p<0.05). r2, determination coefficient.
Figure 2
Apparent viscosity of beef meat emulsions with banana peel albedo powder (BPAP) added at 0.0%, 0.5%, 1.0%, and 1.5% based on the weight of the raw beef used. The actual contents of BPAP in the emulsions were 0.0%, 0.025%, 0.038%, and 0.075% (w/v), respectively.
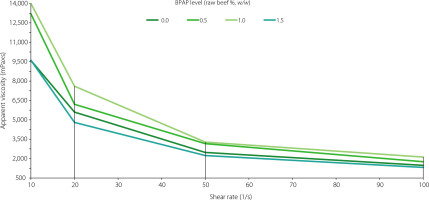
Incorporation of BPAP at different levels affected the AV of the beef meat emulsions (Figure 2). While the addition of BPAP at the levels of 0.5% and 1.0% of raw beef meat (w/w), which corresponded to 0.013% and 0.025% of the emulsion (w/v), respectively, increased AV compared to the control (0.0% BPAP), the addition at the level of 1.5% of raw beef, w/w (0.038% of the emulsion, w/v) decreased AV. The AV of all emulsions diminished with shear rate.
Choi et al. [2009] investigated the quality characteristics of low-fat meat emulsions by replacing pork fat with various vegetable oils (olive, corn, soybean, canola, and grape seed) and rice bran fiber. They stated that AV values of all groups decreased as the rotation period increased. Therefore, the highest viscosity values were found in the meat samples including rice bran fiber.
In addition, Choi et al. [2014] found that the inclusion of makgeolli lees fiber and varying fat contents had an impact on the frankfurter meat batters’ perceived viscosity. All raw batters were reported to be thixotropic, with apparent viscosity decreasing with rotation time. It was also determined that higher levels of fat and macgeolli fiber in the samples caused more pronounced changes in the apparent viscosity of the batters.
Apparent viscosity is an important parameter affecting the quality characteristics of meat, and emulsions with high apparent viscosity are preferred, since high apparent viscosity emulsions are not easily broken [Choi et al., 2009, 2014].
Texture properties of cooked beef meat emulsions
The texture parameters of cooked beef meat emulsions with different contents of BPAP are shown in Table 6. The hardness, adhesiveness, springiness, cohesiveness, and gumminess were in the ranges of 3.65–4.00 N, (−1.80)–(−1.38) N×s, 0.89–0.94, 0.72–0.77, and 2.63–3.04 N, respectively. When increasing the addition level of BPAP from 0.0% to 1.5% of raw beef meat, w/w (which corresponds to 0.0–0.038% in the emulsions, w/v), the hardness of the beef emulsions decreased, while there was a fluctuation in the springiness values. On the other hand, adhesiveness, cohesiveness and gumminess of the emulsions were not significantly (p≥0.05) affected by the addition of BPAP.
Table 6
Textural properties of beef meat emulsions with banana peel albedo powder (BPAP) added at different levels.
The decrease in hardness observed with increasing levels of BPAP in this study is consistent with the findings of Bastos et al. [2014], who reported that the addition of green banana flour to meat burgers resulted in lower hardness compared to the control samples, likely due to the water-binding capacity of the fiber. Similarly, García et al. [2006] found that the incorporation of inulin in a jelly form into sausages led to a reduction in hardness and an improvement in elasticity. However, in contrast to our results, several other studies have reported an increase in hardness following the addition of various dietary fibers. Choi et al. [2007] found that the incorporation of wheat fiber into meat products significantly increased hardness. Additionally, Zaini et al. [2020] showed that banana peel powder increased hardness and chewiness in chicken sausages depending on the inclusion level. These conflicting findings may be explained by differences in the type and structure of the fiber used, its concentration, degree of solubility, and processing method (e.g., fresh or dried). Furthermore, the interaction of dietary fiber with the protein matrix and the fat content of the meat product can also influence textural outcomes. As highlighted by Yadav et al. [2018], such factors are critical in shaping the textural properties of meat products, as they can alter key texture parameters like hardness, springiness, and chewiness depending on the fiber’s characteristics and the processing conditions.
Microstructure of beef meat emulsions
In our research, the effects of BPAP on the microstructural properties of meat emulsions were observed using a CLSM. The CLSM is a suitable method to scan the distribution of oil particles and the interactions between oil and protein. The CLSM micrographs showed that the particle sizes of the emulsions containing BPAP at levels of 0.5% and 1% of raw beef (w/w), corresponding to 0.013% and 0.025% in the emulsions (w/v), were smaller than of the emulsions without banana peel powder and had a more stable, homogeneous appearance (Figure 3). In addition, the oil particles of the emulsion containing 0.025% BPAP were more equally and better dispersed, and there were fewer voids in the emulsion system. Zhao et al. [2019] reported that in the CLSM micrographs, the meat emulsions without regenerated cellulose fiber had considerably larger particles, while the emulsions with higher fiber ratios had smaller particle sizes. In agreement with our results, the emulsion with 0.8% regenerated cellulose fiber had the smallest particle size, and its oil particles were more homogeneously dispersed. Qi et al. [2021] suggested that the citrus fiber was mainly imbibed as well-dispersed particles in the oil-water interface of the emulsion particles, thus stabilizing the emulsion.
Figure 3
Confocal laser scanning microscope micrographs of beef meat emulsions with banana peel albedo powder (BPAP) added at 0.0% (A), 0.5% (B), 1.0% (C), and 1.5% (D) based on the weight of the raw beef used. The actual contents of BPAP in the emulsions were 0.0%, 0.025%, 0.038%, and 0.075% (w/v), respectively.
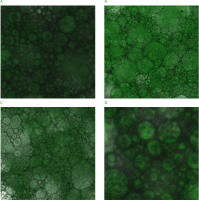
Câmara et al. [2020] reported that the addition of chia mucilage to emulsified meat products caused changes in their structure assessed by CLSM. In the case of 2% chia mucilage powder and 2% chia mucilage gel addition treatments, while a definite grade of cohesion was observed in the protein structures, the fat particles, which had a well-described view, were imaged as looser and less connected in the protein matrix, particularly in the samples added with 2% chia mucilage powder. The authors suggested that this result, seen in the images of the samples with 2% chia mucilage powder added, could be straightly associated with the lower emulsion stability. In the current study, the stability of the beef meat emulsions with BPAP at 0%, 0.5% and 1.5% of raw beef (w/w) was found to be lower than that of the samples with BPAP at 1% of raw beef (w/w). In addition, the CLSM micrographs of these samples with BPAP at 0%, 0.5% and 1.5% of raw beef (w/w) showed they were less bound, looser, and inhomogeneous. Câmara et al. [2020] reported that when the emulsified meat samples contained the double amount of chia mucilage, the protein structures in the CLSM micrographs were more porous, discontinuous, and non-uniform.
Zeeb et al. [2018] found that the use of pectin substantially modified the microstructure of raw fermented and emulsion-type sausages. Other authors also observed that dietary fibers influenced the microstructure of meat [Han et al., 2018]. Tong et al. [2018], using CLSM micrographs, found that konjac glucomannan formed homogeneous gels with myofibrillar protein and showed good conformity with it. The conclusion was that a matrix-like structure was formed.
CONCLUSIONS
The present study results indicate that the physicochemical, emulsification, and microstructural characteristics of meat emulsions could be improved by using of BPAP. The addition of BPAP up to 1.5% of raw beef (w/w) level, corresponding to content of 0.038% (w/v) in the emulsion, increased the WHC and decreased the CL, hardness, and chewiness, which could be due to the presence of dietary fiber in the banana peel. However, the incorporation of BPAP up to 1.0% of raw beef (w/w) increased the emulsion capacity, emulsion stability values, and the AV of the samples. Regarding the CLSM micrographs, BPAP up to 1% of raw beef (w/w) (0.025% in the emulsion, w/v) led to more stable and homogenous meat emulsions. Consequently, it is recommended to use BPAP as a cheap source of dietary fiber in meat emulsions at a level of 1% of raw beef (w/w) (0.025% in the emulsion, w/v) to improve technological, textural, and microstructural properties. Further studies should be undertaken to clarify the effect of banana peel albedo powder on the microbiological stability and bioactive potential of meat products.

