INTRODUCTION
Today, despite its many advantages for human nutrition, meat is considered a less sustainable food choice due to growing concerns about health and the environment. Recently, many changes in dietary recommendations have been noticed, focusing much attention on the heavy environmental burden of meat production. In Central and Western Europe, there is a strong tradition of meat consumption, but increased health awareness has led to a demand for alternative, low-fat, and antibiotic-free types of meat [Hoffman & Wiklund, 2006; Niewiadomska et al., 2020]. In addition, consumers are looking for balanced and nutritious meat that can be included in a climate-friendly diet. When estimating the carbon footprint of farmed meat, many relative factors must be considered, ranging from feeding to energy, transportation, and slaughter. Therefore, the calculations for farmed meat are much higher than those for free-living game meat. For consumers who like meat, a pro-climate choice can be venison. In Poland, the most common species of deer is roe deer. Its meat is characterised by a unique taste and aroma but also by high quality and unique dietary and health-promoting properties. Roe deer meat is high in protein, unsaturated fatty acids, iron, and low in fat and saturated fatty acids [Cygan-Szczegielniak & Janicki, 2012a; Zomborszky et al., 1996]. The high content of polyunsaturated fatty acids (PUFAs) is characteristic of the muscle tissue of game animals and much higher than that of farm animals [Klupsaite et al., 2020; Razmaitė et al., 2015]. Importantly, deer meat is free from antibiotics and growth and development enhancers, and animals are not exposed to the stress of commercial breeding [Cygan-Szczegielniak & Janicki, 2012b; Zomborszky et al., 1996]. Another important aspect is the higher vitamin, folate, and mineral content of venison meat compared to many farmed species [Królikowski & Deptuła, 2020]. Despite its many advantages, the consumption of venison in Poland remains surprisingly low compared to pork, poultry, and beef. It is also significantly lower than in other European countries [Cygan-Szczegielniak & Janicki, 2012b; Królikowski & Deptuła, 2020; Kwiecińska et al., 2018].
Venison meat and meat products obtained in Poland are largely exported to the markets of Western Europe; meanwhile, conditions in Poland could successfully contribute to an increase in the share of venison in the meat industry [Królikowski & Deptuła, 2020]. The high price of game meat and problems in maintaining quality during carcass processing and storage are the main reasons for low consumption [Kwiecińska et al., 2018]. Most of the research carried out on the storage of meat has focused on the meat of farm animals [Daszkiewicz et al., 2008; Vergara et al., 2003]. The selection of suitable storage conditions and time plays an important role from the point of view of both consumer acceptance and the meat industry. However, incorrect storage can directly affect the deterioration of raw material quality [Kwiecińska et al., 2018]. Meat is rich in many nutrients, but these compounds are prone to degradation processes, including oxidative ones especially in the case of proteins and lipids [Domínguez et al., 2019].
Monitoring oxidative processes in food systems is important from the perspective of human nutrition and is therefore crucial for the meat industry [Lund et al., 2011; Soladoye et al., 2015]. Oxidative processes result in the deterioration of meat’s functional properties and negatively affect its nutritional value [Estévez, 2021; Kasałka-Czarna et al., 2022; Li et al., 2020; Soladoye et al., 2015]. In addition to a reduction in the nutritional quality and bioavailability of oxidised proteins, the consumption of these modified proteins may be associated with an increased risk of certain disease states [Estévez & Xiong, 2019; Lund et al., 2011; Soladoye et al., 2015; Thøgersen et al., 2020]. Soladoye et al. [2015] conducted a comprehensive review of the oxidative processes of proteins in processed meat, which highlighted the impact of the consumption of oxidised proteins in ageing and diseases associated with oxidative modifications of proteins and included Alzheimer’s disease, kidney failure, and diabetes, among others. Protein oxidation results in the formation of oxidised muscle protein products, but the characteristics of these products are highly dependent on the amino acids involved and mechanisms of oxidation process initiation. The oxidative changes of proteins include modifications of amino acid side chains, fragmentation, aggregation and polymerisation of proteins Oxidative changes in proteins can be monitored by measuring the content of formed carbonyl groups [Estévez, 2011; Soladoye et al., 2015]. Scientific studies have shown that processing and storage influence the development and intensity of oxidation processes in farmed species [Lund et al., 2011]. In the case of game animals, which are characterised by a higher content of iron, myoglobin, and polyunsaturated fatty acids, the susceptibility to oxidation increases [Chaijan, 2008]. Despite this, there is still a scarcity of scientific research on oxidative processes within wild game meat. The main non-microbiological cause of meat deterioration is lipid oxidation, which is one of the main factors affecting meat quality, acceptability, and shelf life [Domínguez et al., 2022]. Oxidative processes begin at slaughter and then intensify during processing, handling, and storage. The formation of lipid oxidation products results in discolouration, unpleasant odour and taste, and changes in texture [Chaijan, 2008]. In addition to reducing the nutritional value of meat, the aldehydes and oxysterols formed upon lipid oxidation exert pro-inflammatory, cytotoxic, and mutagenic effects [Domínguez et al., 2022; Sottero et al., 2019]. As Chaijan [2008] suggested, lipid oxidation leads to the formation of a wide range of aldehyde products that induce the oxidation of oxymyoglobin, resulting in an increase in the peroxidative activity of metmyoglobin. Oxidation processes can be effectively controlled by using appropriate packaging, storage or preservation technologies [Bao & Ertbjerg, 2019; Moczkowska et al., 2017; Wazir et al., 2019].
The objective of this study was to use different storage methods and storage times for venison to preserve its quality (pH, colour, tenderness, water activity, water loss during cooking and storage) and to inhibit protein and lipid oxidation. Identifying differences in biochemical processes and quality of venison may allow improving production and packaging techniques and changing nutritional strategies for this type of meat. These factors have already been evaluated for common slaughter species, whereas studies on roe deer are still lacking.
MATERIALS AND METHODS
Sampling
Samples for the research were obtained from five carcasses of roe deer (Capreolus capreolus). Animals were hunted in Hunter District No. 57, OHZ, Sarbia Forest District (Poland). The selection was based on similarity of sex (male) and age (2–4 years), verifying the development of the dentition. Shooting and evisceration were performed under hunting protocol (internal carcass inspection report based on Regulation (EC) No 853/2004). The carcass was then cooled and stored at 0°C for 5 days at the Venison Buying Station (Drzązgowo Forestry, Sarbia Forest District). This procedure is an ageing procedure, which is longer for game meat than for slaughtered farm animals. Carcasses were assessed after skinning to exclude meat with defects (damaged elements by shooting, inappropriate pH value or improperly cooled meat). From each skinned roe deer carcass, three muscles were excised without bone and meat on the bone, namely musculus longissimus thoracis et lumborum (LTL), musculus biceps femoris (BF), and musculus vastus lateralis (VL). The cuts were vacuum-packed and transported to the Department of Meat Technology, Poznan University of Life Sciences, Poland in a portable car refrigerator with a permanent power supply (18445, Norauto, Warsaw, Poland). The muscles (without bone) were divided into equal, selected parts (approximately 80 g). Muscles (in triplicate) were vacuum-packed (VAC) in barrier bags (MULTIVAC Seep Haggenmüller SE & Co. KG, Wolferschwenden, Germany) and in a modified atmosphere (MAP) (80% O2, 20% CO2) (Multivac T200 packaging machine) and sealed with polyethylene terephthalate/polyethylene (PET/PE) film. Samples were stored at 4±1°C for 21 days. Muscles on the bone were stored in a dry-ageing cabinet (DRY-AGED) at 2°C and 80% humidity for 21 days (Dry-Aged Cooler SW-28, Arnsberg, Germany). Samples were analysed immediately after preparation (0 days) and on the day 7 and 21 of storage, for each variant.
Proximate composition analysis
The proximate composition (protein, moisture, fat, and ash contents) was determined in triplicate for each muscle on fresh meat before packing. The composition was evaluated according to ISO standards. The protein content was calculated based on total nitrogen content and the 6.25 conversion factor for meat and dairy products following ISO 937:1978. In turn, respective Polish Standards: PN-ISO 1442:2000, PN-ISO 1444:2000, and PN-ISO 936:2000, were used for the other parameters tested. Samples for analysis were homogenised using a food processor (Moulinex S 643.23, Paris, France).
Analysis of the composition of minerals
Ash of raw meat samples (0.500±0.001 g) was digested with 5.0 mL of 65% HNO3 (Merck, Darmstadt, Germany) in closed Teflon vessels (55 mL) at 180°C using the Mars 6 microwave digestion system (CEM, Matthews, NC, USA). Digested samples were diluted with high-pure deionized water (≥18 MΩ×cm resistivity) obtained from Milli-Q water purification system (Merck Millipore, Burlington, MA, USA) to a final volume of 10 mL. The procedure was reproduced after Zubaidi et al. [2021]. Contents of 13 minerals (K, Na, Mg, Ca, Zn, Fe, Al, Cu, Pb, Cd, Cr, Mn, and Ni) were determined in the samples with the inductively coupled plasma high-resolution optical emission spectrometry (ICP-HROES), using a PlasmaQuant PQ 9000 Elite spectrometer (Analytic Jena, Germany). The settings were as follows: radio frequency (RF) power 1.2 kW, auxiliary gas flow 0.5 L/min, nebulizer gas flow 0.5 L/min, plasma gas flow 12 L/min, and axial plasma view. High-resolution optics were echelle double monochromator and charge-coupled device (HR CCD), which was cooled to −10°C using the Peltier system. The following wavelengths of emission lines were applied: K 769.897 nm, Na 588.995 nm, Mg 285.213 nm, Ca 315.887 nm, Zn 206.200 nm, Fe 238.204 nm, Al 396.152 nm, Cu 324.754 nm, Pb 220.353 nm, Cd 214.441 nm, Cr 267.716 nm, Mn 257.610 nm, and Ni 231.604 nm. Acquisition time was 1 s and signal was measured in 5 replicates. Argon was used as the internal standard (Ar 420.068 nm). Detection limits (DLs) were estimated at the level of 0.03 mg/kg dry weight (DW). ICP commercial analytical standards (Romil, Cambridge, UK) were used for calibration. Standard addition method was used in the quality control with acceptable recovery (80–120%). The uncertainty for the complete analytical process (including sample preparation) was at the level of 20%.
Water activity, pH and colour measurements
The water activity, pH and colour indices were measured on fresh meat before packing and on stored meat for 7 and 21 days under VAC, MAP, and DRY-AGED treatment. The water activity measurement was performed using a LabMaster-aw neo instrument (Novasina AG, Lachen, Switzerland) at 25±1°C. The meter was calibrated with standard SAL-T 97% tagged with an automatic recognition system (RFID). The pH values were measured at room temperature (22–24°C) with a portable Handylab 2 pH meter equipped with an L68880 pH combination electrode (Schott AG, Mainz, Germany). The pH buffers 4.01 and 7.00 (Testo Ltd., Alton Hampshire, UK) were used for calibration. The colour on the cross-section of freshly cut samples was measured three times by the CIELab colour space using a Dr. Lange LMG161 portable colorimeter (Dr. Bruno Lange GmbH & Co. KG, Berlin, Germany). The values of lightness (L*), greenness/redness (a*), and blueness/yellowness (b*) were measured. The setting parameters were as follows: illuminant D65, aperture size of 5.0 mm, and observer angle of 10°. The instrument was standardised using a white standard (no. 3125).
Purge and cooking losses determination
The purge and cooking losses were expressed as a percentage, by weighing the samples before and after thermal treatment or the storage period. The purge losses were determined after 7 and 21 days of storage for each storage method while cooking losses were also determined for fresh meat. To determine cooking losses, 25 mm slices of meat wrapped in aluminium foil were heated to a core temperature of 72°C in a Rational combi model SCC 61 convection oven (Landsberg am Lech, Germany) at a temperature of 160°C and a 75% humidity in the cooking chamber. Cooking losses were determined on cooled samples at room temperature.
Warner-Bratzler shear force measurement
The samples cooked and cooled according to the procedure for cooking losses determination were cut into cuboids (1×1×3 cm). The Warner-Bratzler shear force (WBSF) for every muscle was tested before packing and after 7 and 21 days of storage (VAC, MAP, DRY-AGED). WBSF was measured by a TA.XT.plus texture analyser (Stable Micro Systems, Godalming, UK) using a WarnerBratzler add-on device. The shear force expressed as N was measured perpendicularly to the arrangement of muscle fibres at a traverse speed of 100 mm/min.
Protein carbonyl content determination
Protein oxidation was determined by the total carbonyl content using 2,4-dinitrophenyl hydrazine (DNPH) derivatisation method according to Levine et al. [1994], with slight modifications. Fresh samples and packed meats (VAC, MAP, DRY-AGED) stored for 7 and 21 days were subjected to the analysis. One gram of meat was homogenised (Ultra Turrax) in 20 mM sodium phosphate buffer containing 6 M NaCl (pH 6.5) for 30 s, and immediately filtered through filter paper. Samples from homogenate (two aliquots of 0.4 mL) were precipitated by cold 10% trichloroacetic acid (TCA) (1.5 mL) and incubated at 20°C for 20 min. After incubation, the samples were centrifuged for 5 min at 4°C at 10,000×g. One of the pellets was treated with 1 mL of 2 M HCl (for the protein content measurement), the second with 1 mL of 10 M DNPH in 2 M HCl (for the carbonyl content measurement), and then were incubated for 30 min at 37°C. Afterward, the samples were treated with 1 mL of 10% TCA and centrifuged for 5 min at 4°C and 10,000×g. To remove excess DNPH after centrifugation, the precipitates were washed 3×1 mL with a solution of ethanol and ethyl acetate (1:1, v/v). The obtained pellets were then dried at room temperature for 1 h. The dried samples were dissolved in 1.5 mL of 20 mM sodium phosphate buffer containing 6 M guanidine hydrochloride (pH 6.5) and incubated for 30 min at 37°C. To remove insoluble fragments, the samples were centrifuged for 5 min at 4°C at 10,000×g. The absorbance was measured (Ultrospec 2000, Pharmacia Biotech, Uppsala, Sweden) at 280 nm (A280) and 370 nm (A370) and carbonyl content (nmol mg protein) was calculated using Equation (1) [Levine et al., 1994]:
Determination of thiobarbituric acid reactive substance content
The thiobarbituric acid reactive substance (TBARS) content of LTL, BF, and VL was assessed according to the method described by Lo Fiego et al. [2004], with minor modifications. The analysis was performed for fresh samples and in meat stored (VAC, MAP, DRY-AGED) for 7 and 21 days. Raw meat samples from each muscle (10 g) were homogenised in 20 mL of 20% TCA at 10,000×g for 60 s and then centrifuged at 3,502×g for 15 min at 4°C. The supernatant was filtered through qualitative filter paper by vacuum filtration. The volume of 5 mL of 20 mM 2-thiobarbituric acid was added to 5 mL of the filtrate. The blank was 5 mL of TCA and 5 mL of 2-thiobarbituric acid solution. Solutions were heated for 20 min in a boiling water bath and afterward cooled to 21°C. Absorbance was measured using an Ultrospec 2000 spectrophotometer (Pharmacia Biotech) at 532 nm. Results were expressed as mg of malonic dialdehyde (MDA) equivalents per kg of meat (mg MDA/kg).
Statistical analysis
Statistical analysis of the results was performed using Statistica 13.1 (StatSoft Inc., Tulsa, OK, USA). The statistics were consistent with the assumption of a normal distribution (verified by the Shapiro-Wilk test). One-way and two-way analysis of variance (ANOVA) was used. Tukey’s Honestly Significant Difference (HSD) test was used to assess the significance of differences. The influence of the storage method of LTL, BF, and VL samples, as well as storage period up to 21 days on the quality characteristics, were assessed. The results were considered to be statistically significant at p<0.05, and expressed as mean ± standard error of mean (SEM).
RESULTS AND DISCUSSION
The proximate composition, physicochemical properties, and shear force
The proximate composition of the three muscles tested (LTL, BF, VL) is shown in Table 1. Based on ANOVA, it was found that the type of muscle significantly (p<0.05) affected the chemical composition (protein and fat content). The protein content of roe meat was 216.5–228.6 g/kg, while the fat content was 17.1–25.8 g/kg. Roe deer meat can therefore be categorised as low-fat meat. The muscle containing the most protein and the least fat was LTL. The protein content of VL and BF muscles was lower, while the fat content was higher than that of LTL. There were no statistically significant differences in moisture and ash contents between the muscles.
Table 1
Proximate composition of roe deer muscles: longissimus thoracis et lumborum (LTL), biceps femoris (BF), and vastus lateralis (VL).
| Component (g/kg) | LTL | BF | VL | p |
|---|---|---|---|---|
| Protein | 228.6 ± 1.1a | 217.3 ± 1.3b | 216.5 ± 1.7b | *** |
| Moisture | 738.9 ± 1.6 | 744.4 ± 2.1 | 742.6 ± 2.1 | NS |
| Fat | 17.1 ± 1.0b | 22.6 ± 1.0a | 25.8 ± 0.8a | *** |
| Ash | 14.1 ± 1.6 | 15.6 ± 1.7 | 15.0 ± 2.0 | NS |
According to Ivanović et al., [2020], the protein content of LTL from roe deer was 21.40%, while the fat content was 1.31%. Other authors [Daszkiewicz et al., 2018] showed a similar protein content in this muscle, i.e. 229.76 g/kg, to that determined in the present study. As reported by Cygan-Szczegielniak & Janicki [2012a], the age of the animal had a direct effect on the proximate composition of the muscle derived from it. LTL muscle obtained from roe deer aged 2–3 had the highest protein content (24.02%) compared to the other age groups (4–5 and 6–7 year old), and a low fat content (0.43%). According to other studies, the fat content of roe deer in LTL ranged from 0.36 to 1.76% and depended on the season [Dominik et al., 2012]. Compared to wild boar meat, which is also a representative of game, roe deer meat is richer in protein and has a lower fat content [Kasałka-Czarna et al., 2022]. Nevertheless, the proximate composition of game meat is strongly influenced by diet, season, hunting, habitat, sex, and animal activity [Ivanović et al., 2020].
Table S1 (in Supplementary Materials) shows the content of selected minerals in the LTL, BF, and VL. The highest contents were determined for potassium, sodium, magnesium, zinc and iron. The content of iron ranged from 14.8 to 24.6 mg/kg of meat. No heavy metals, such as cadmium, chromium, manganese, or nickel, were found. Roe deer meat was richer in potassium, magnesium, iron, and phosphorus than that of wild boar [Kasałka-Czarna et al., 2022; Zomborszky et al., 1996].
The physicochemical properties of muscles of roe deer (fresh and stored for 7 and 21 days in VAC, MAP and DRY-AGED variant) are presented in Table 2. Their values indicate a significant effect of storage time and method as well as their interaction on the pH of the tested muscles (Table 3). The pH values of fresh meat ranged from 5.53 to 5.63, depending on muscle type. Of all the roe deer muscles, VL had the highest pH for each storage method. Significantly higher pH values, compared to the other methods, were observed for DRY-AGED muscles on both day 7 and 21 of storage (pH ranged from 5.53 to 5.74 for LTL), while the pH of the muscles stored in MAP on day 7 was similar to the pH of the VAC samples, with exception of VL. On day 21, the highest decrease in pH was noted for BF in MAP (5.44). The VAC muscles featured the greatest fluctuations in pH values. Similar relationships between the effect of storage method and time were shown for the wild boar muscles in a similar experiment, but MAP storage resulted in a significant decrease in pH for each wild boar muscle (compared to 5.23 for BF) [Kasałka-Czarna et al., 2022]. According to Daszkiewicz et al. [2018], the pH value measured 48–54 h post-mortem in LTL of roe deer was 5.52. However, for the LTL taken 24 h post-mortem, the pH was 5.72 [Ivanović et al., 2020]. Other researchers have also shown that the pH of fresh meat from roe deer measured 120 h post-mortem ranged from 5.52 to 5.56, depending on the muscle metabolism [Dominik et al., 2012]. A characteristic of game meat is the high content of lactic acid produced by glycolysis, which influences meat acidity. The pH values of venison meat measured up to 96 h post-mortem ranged from 5.4 to 5.6. These values also changed according to season, sex, and age [Dominik et al., 2012]. Storage time resulted in differences in meat pH. Vergara et al. [2003] studied deer meat stored in a modified atmosphere in three gas mixtures (40% CO2 + 60% N2, 80% CO2 + 20% O2, and 80% CO2 + 20% N2). They noted that storage time affected the increase in pH for each method. In turn, Gill [1990], found that the pH of lamb meat decreased when it was packaged in a CO2-rich atmosphere. Thus, depending on a given species, meat undergoes different changes with the same gas atmosphere in packaging.
Table 2
Physicochemical properties of roe deer muscles: longissimus thoracis et lumborum (LTL), biceps femoris (BF), and vastus lateralis (VL) during storage under modified atmosphere (MAP), dry (DRY-AGED) and vacuum (VAC) ageing.
[i] Results are shown as mean ± standard error of mean (SEM). Means with different superscripts a–c in a column for a given parameter differ significantly within storage time (p<0.05). Means with different superscripts x–z in a row for a given muscle differ significantly within storage methods (p<0.05). p-Time, effect of time within method; p-Method, effect of method within time; *p<0.05; **p<0.01; ***p<0.001; NS, non-significant (one-way ANOVA). L*, lightness; a*, greenness/redness; b*, blueness/yellowness.
Table 3
Effect of time (0, 7, 21 days) and method of storage (dry, vacuum, and modified atmosphere ageing) and their interaction on selected properties of roe deer muscles: longissimus thoracis et lumborum (LTL), biceps femoris (BF), and vastus lateralis (VL).
The study demonstrated a significant effect of storage time and method as well as their interaction on the lightness values (L*) of the BF and VL (Table 3). For VL, there was a weaker interaction between storage method and storage time than for BF. The value of L* color parameter of meat on day 21 of MAP and VAC storage increased compared to the fresh samples (Table 2). For a* and b* values, an interaction was demonstrated between the storage method and time for each muscle (Table 3). Time did not significantly affect a* values of BF and VL and b* values of BF. The storage method also had no significant effect on a* value of LTL. MAP had a negative effect on the a* parameter (Table 2). Its value increased in DRY-AGED BF, whereas remained unchanged after 21-storage in VAC. On day 21 of the analysis, significantly lower a* values were determined in the samples stored in MAP compared to the other two methods. This is most likely due to the formation of oxymyoglobin (OxyMb) under aerobic conditions in an atmosphere with a high oxygen concentration [Chaijan, 2008]. The samples stored in VAC and DRY-AGED had significantly lower values of yellowness (b*) compared to the meat stored in MAP (Table 2). Vergara et al. [2003] found that the b* value in deer meat increased when the O2 content of the package was higher. The sharp changes in the b* parameter values suggest that this gas was responsible for the browning of red deer meat. Oxygen is used to enhance fresh meat colour, as it maintains the characteristic red colour of oxymyoglobin, which is the oxidised form of myoglobin. However, at increased concentrations, oxygen can negatively affect meat colour [Świderski & Sadowska, 2011]. The increase in the b* value observed during meat storage may be due to metmyoglobin formation [Daszkiewicz et al., 2011]. Myoglobin is involved in the storage of oxygen in red muscles. The addition of oxygen can retard its formation, but when oxygen-free packaging is used, intense oxidative changes occur, which can contribute to the formation of its large amounts [Świderski & Sadowska, 2011]. A similar effect can be observed with vacuum packaging when oxygen is removed from the meat surface, which can also result in a change in meat colour towards browning. According to Daszkiewicz et al. [2011], no significant differences were found between the average values of the colour indices of roe deer meat after 7 days of storage in the case of using vacuum packaging and MAP (40% CO2 + 60% N2; 60% CO2 + 40% N2). The results also showed that, after 21 days of storage, the use of MAP with 60% N2 had the smallest effect on colour changes. Other researchers have noted a similar relationship for deer meat [Vergara et al., 2003]. The pH value also affects L* values, and a lower pH increases protein denaturation, resulting in higher L* values [Klupsaite et al., 2020].
A significant effect of storage time and method on water activity (aw) was shown for all muscles (Table 3). However, no significant interaction between method and storage time was observed for LTL. On day 7 of MAP storage, aw values for all muscles were lower (0.9675 for LTL, 0.9690 for BF, and 0.9686 for VL) compared to fresh meat and meat stored for 21 days (Table 2). No similar relationship was observed for VAC and DRY-AGED. After 21 days, a significant decrease in aw was determined in DRY-AGED meats, e.g., from 0.9754 to 0.9590 for LTL. This decline corresponds to the highest purge losses (Figure 1). The highest aw was determined for the samples stored for 21 days in MAP, especially for LTL (0.9737). For vacuum packaging, the decrease in water activity was not significant after longer storage. Meat seasoning had a positive effect on aw, which was also observed for wild boar meat [Kasałka-Czarna et al., 2022]. The growth of microorganisms and the rate of biochemical processes are strongly dependent on water activity. Additionally, according to Florek et al. [2019], DRY-AGED meat has a lower water content than those stored in VAC, which affects, among others, its microbiological safety during storage. According to other reports, for the same storage conditions (MAP, VAC, DRY-AGED), at 7, 14 and 21 days of storage, wild boar meat was microbiologically safe [Kasałka-Czarna et al., 2022].
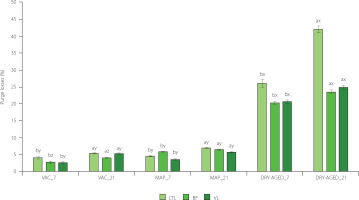
Figure 1
Purge losses in muscles longissimus thoracis et lumborum (LTL), biceps femoris (BF) and vastus lateralis (VL) of roe deer during storage (at day 7 and 21) under modified atmosphere (MAP), dry (DRY-AGED) and vacuum (VAC) ageing. Results are shown as mean and standard error of mean (SEM). Different a and b letters above bars indicate a significant effect of time within the method (p<0.05). Different x–z letters above bars indicate a significant effect of the method within time (p<0.05).
In terms of cooking losses, the interaction effect of storage time × storage method (Table 3) on LTL, BF and VL was significant (p<0.001). Significant effect of time and storage method was found for BF and VL (p<0.001; p<0.001) and LTL (p<0.01; p<0.001), respectively. Cooking losses in fresh meat were 23.03% in LTL, 28.52% in BF, and 22.45% in VL (Table 2). The lowest percentage of thermal loss after 7 days of storage was noted in the DRY-AGED (21.10% for LTL, 30.00% for BF, and 23.28% for VL) and VAC (22.59% for LTL, 29.31% for BF and 23.03% for VL) muscles. For VAC, cooking losses significantly increased on day 21 of storage relative to the fresh sample for VL and BF. Significant differences were noted at day 7 of MAP storage for LTL and VL compared to both the fresh sample and the sample stored for 21 days. The increase of the thermal losses for MAP stored samples indicates a decrease in water retention in the muscle, which may have a direct effect on its texture. This was not observed for VAC in muscles from wild boar, in which cooking losses decreased during storage [Kasałka-Czarna et al., 2022]. According to Daszkiewicz et al. [2011], roe deer meat stored (m. longissimus dorsi) under vacuum was characterised by increased cooking losses compared to the modified atmosphere with oxygen exclusion. Despite this, no significant differences were observed in cooking losses between MAP and VAC. Therefore, the composition of the modified atmosphere can have an impact on cooking losses. In the early phase of storage, a characteristic crust develops on the surface of DRY-AGED meat due to water loss [Dashdorj et al., 2016]. The increase in water retention capacity is strongly correlated with an increase in meat pH. As a result of the increase in pH, the protein structure became more open and myofibrillar proteins could bind more water. Despite high storage losses and high water loss, DRY-GED meat with the highest pH is of high quality [Dashdorj et al., 2016]. The same result was obtained from wild boar meat analyses (on day 21 of storage of LTL, pH values were 5.31 (MAP), 5.46 (VAC), 5.72 (DRY-AGED), and cooking losses were 30.82%, 30.92%, 20.94%, respectively) [Kasałka-Czarna et al., 2022].
Significant (p<0.001) effect of storage time and storage method on purge losses was found in the study (Table 3). The interaction effect of storage time × storage method (Table 3) on LTL, BF and VL was significant (p<0.001 for LTL and BF; p<0.01 for VL). Purge losses were highest on day 21 of storage for each muscle and storage method (Figure 1, Table S2). The lowest purge losses were determined for MAP and VAC. For LTL on day 21 of storage, there was an increase of 2.3% for MAP and 1.27% for VAC relative to day 7 of storage. For LTL DRY-AGED, the difference was 15.92% (26.12% on day 7 and 42.04% on day 21).
After 7 and 21 days of storage, the purge losses of DRY-AGED meat due to drying and water loss were significantly four to even seven times higher. Daszkiewicz et al. [2008] reported lower losses for roe deer LTL in VAC (1.50% after 7 and 2.17 after 21 days of storage) and observed that the use of modified atmosphere packaging with 60% CO2 + 40% N2 resulted in reduced purging. The extent of purge losses can have some economic consequences, as, e.g., DRY-AGED storage of roe deer meat dramatically reduces the overall process efficiency. A higher storage loss was recorded within each method for roe deer compared to wild boar meat [Kasałka-Czarna et al., 2022].
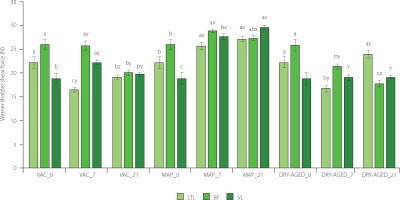
The study demonstrated a significant (p<0.001) effect of storage time and method as well as their interaction on the WBSF of the LTL, BF and VL (Table 3). VAC and DRY-AGED had a positive effect on tenderness at day 21 of storage (except for LTL, DRY-AGED and VL), whereas MAP adversely affected this quality attribute during meat storage (Figure 2, Table S2). For LTL muscle stored as DRY-AGED, the lowest values were reached on day 7 of storage (16.91 N). For each of the MAP muscles, WBSF values increased on day 21 relative to the fresh sample. The highest tenderness of BF and VL muscles was reached after 21 days of storage for DRY-AGED (17.83 N and 19.08 N, respectively). For LTL, the lowest values were observed on day 7 of storage for both DRY-AGED and VAC (16.91 N and 16.53 N, respectively). Klupsaite et al. [2020] showed that roe deer meat analysed 24 h post-mortem had the highest tenderness compared to other game species (wild boar, deer, and beaver), and its tenderness was satisfactory (1.17 kg/cm2 for m. glutenus medius). In another study, cutting force was determined for LTL from roe deer 24 h post-mortem at 47.21 N [Dominik et al., 2012]. Therefore, the carcass skin maturation treatment applied in this study for 5 days had a major effect on the tenderness of roe deer meat. The muscles showed satisfactory tenderness on the day the samples were obtained. According to Daszkiewicz et al. [2008], in LTL from roe deer, VAC storage for 21 days had a positive effect on tenderness (16.24 N). The researchers used modified atmosphere packaging, but with the elimination of oxygen (40% CO2 + 60% N2 and 60% CO2 + 40% N2); the analyses showed a positive effect on tenderness development. Other researchers have also reported that high oxygen concentrations may contribute to increased WBSF. This may be due to increased oxidation of proteins and fats, resulting, for example, in the formation of oxygen-induced protein aggregates [Clausen et al., 2009; Moczkowska et al., 2017]. In pork and beef stored in a high-oxygen atmosphere, myosin heavy chains form intermolecular bonds that are not observed without the presence of oxygen. This may contribute to the deterioration of tenderness [Lund et al., 2007]. In addition to the method and time of storage, these differences can also be influenced by different muscle metabolisms. In another study on beef stored in a high-oxygen atmosphere, a reduction in tenderness was confirmed due to cross-linking of myosin through disulphide bond formation [Kim et al., 2010].
Figure 2
Warner–Bratzler shear force determined for muscles longissimus thoracis et lumborum (LTL), biceps femoris (BF) and vastus lateralis (VL) of roe deer during storage (at day 0 (fresh), 7 and 21) under modified atmosphere (MAP), dry (DRY-AGED) and vacuum (VAC) ageing. Results are shown as mean and standard error of mean (SEM). Different a–c letters above bars indicate a significant effect of time within the method (p<0.05); Different x–z letters above bars indicate a significant effect of the method within time (p<0.05).
Protein and lipid oxidation
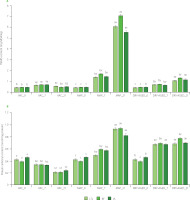
Two-way ANOVA showed the significant effect of storage time and method and the effect of storage time × storage method (Table 2) on protein and lipid oxidation in LTL, BF and VL (p<0.001). During VAC storage, the content of protein carbonyl groups decreased in all muscles at both 7 and 21 days of storage compared to the fresh sample (Figure 3, Table S3). On day 21 of VAC storage, the protein carbonyl content ranged from 0.21 to 0.26 nmol/mg protein. On day 7 of storage, the highest levels of carbonyls were determined for all DRY-AGED muscles. They remained stable, and no considerable increase was observed on day 21, except for BF. Oxidative changes in MAP-stored muscles up to day 7 were less intense than in the DRY-AGED muscles. On day 21 of analysis, however, the increase in protein oxidation observed in MAP samples was the highest, i.e., 121.43% for LTL, 141.03% for BF, and 78.26% for VL, compared to the fresh samples.
Figure 3
Contents of thiobarbituric acid reactive substances, TBARS (A) and protein carbonyls (B) in muscles longissimus thoracis et lumborum (LTL), biceps femoris (BF) and vastus lateralis (VL) of roe deer during storage (at day 0 (fresh), 7 and 21) under modified atmosphere (MAP), dry (DRY-AGED) and vacuum (VAC) ageing. Results are shown as mean and standard error of mean (SEM). Different a–c letters above bars for a given parameter indicate a significant effect of time within the method (p<0.05). Different x–z letters above bars for a given parameter indicate a significant effect of the method within time (p<0.05). MDA, malonic dialdehyde.
As a result of protein oxidation, carbonyl group content for all roe deer muscles within each method did not exceed 0.94 nmol/mg protein (21 days MAP, BF). In a similar study on wild boar meat, the carbonyl contents for fresh LTL, BF, and VL muscles were significantly higher (1.26, 1.15, and 0.86 nmol/mg protein, respectively), while MAP storage contributed to protein oxidation products of 2.48–3.29 nmol/mg [Kasałka-Czarna et al., 2022]. As reported by Lindahl et al. [2010], greater protein carbonylation in beef muscle was observed with high-oxygen packaging. In other studies, carbonyl levels in pork LTL stored for 7 days in MAP with a high oxygen content (70% O2 + 30% CO2) did not change significantly and were slightly higher than for VAC samples. After 14 days, a decrease in carbonyl levels was noted for VAC and a slight increase for MAP samples [Lund et al., 2007]. As reported by Wang et al. [2019], 7 days of pork storage in MAP (80% O2 + 20% CO2) resulted in enhanced (reaching approximately 2.40 nmol/mg protein) formation of protein carbonyls.
The formation of protein carbonyls may also be promoted by a decrease in pH [Estévez, 2011]. In the current study, it was noted that on day 21 of MAP storage, a significant increase in protein oxidation was consistent with a decrease in pH. A deterioration in tenderness, colour, and odour of MAP-stored roe meat was observed in the present study. There are many hypotheses on the consequences of protein oxidation on meat flavour due to the formation of carbonyl groups [Lund et al., 2011]. According to Ventanas et al. [2007] and Fuentes et al. [2010], the formation of protein carbonyls can affect the taste and aroma of meat. In contrast, other authors demonstrated the effect of protein oxidation on changes in meat colour [Domínguez et al., 2019; Estévez & Cava, 2004]. This may explain the changes observed in our study for the b* parameter values of meat during storage in MAP.
The levels of TBARS in fresh roe meat were 0.44, 0.47, and 0.46 mg MDA/kg for LTL, BF, and VL, respectively (Figure 3, Table S3). Daszkiewicz et al. [2018] reported 0.92 mg MDA/kg for LTL roe deer, but Klupsaite et al. [2020] detected 0.30 mg MDA/kg for m. gluteus medius. These values are strongly dependent on muscle metabolism and proximate composition. After 7 days of storage, an increase in lipid oxidation was detected in all samples. Significantly higher TBARS values were noted (after 7 and 21 days of storage) in the samples stored under high oxygen conditions (MAP). The least changes in lipids after 21 days of storage were observed in the samples stored in VAC. In the DRY-AGED muscles, the changes, although significant, occurred more slowly compared with MAP. TBARS values in roe deer meat increased faster than the protein carbonyl content during prolonged storage in MAP (an average increase of 1263% for TBARS and 155% for carbonyls on day 21). In our previous study, we observed that changes within TBARS occurred equally rapidly upon the storage of wild boar meat in a high-oxygen atmosphere [Kasałka-Czarna et al., 2022].
Interestingly, TBARS values were not considerably different for roe deer meat compared to wild boar meat. The LTL from wild boar on day 21 of MAP storage contained 6.15 mg MDA/kg, while the LTL from roe deer had 6.07 mg MDA/kg. High TBARS values result in a rancid aroma or noticeable colour change [Domínguez et al., 2019].The acceptable MDA content for meat products, where no rancid aroma is perceptible, is 2.0–2.5 mg MDA/kg meat [Campo et al., 2006]. Importantly, reducing the oxygen in packaged pork results in the inhibition of both pork protein and lipid oxidation compared with 80% oxygen atmosphere. When the pork was stored in the air, the carbonyl and TBARS contents were lower than in high oxygen MAP [Wang et al., 2019]. Similarly, beef steaks stored in a high oxygen atmosphere (80% O2 + 20% CO2) had higher TBARS values compared to the VAC-packed ones [Łopacka et al., 2016].
CONCLUSIONS
In conclusion, a significant effect of storage time and method (dry, vacuum, and modified atmosphere ageing) of muscles of roe deer on pH, water activity, thermal loss, storage loss, WBSF and lipid and protein oxidation was found in the present study. A significant interaction effect of storage time × storage method was also observed for all traits examined in BF and VL. For LTL, no significant interaction effect was found only for L* values and water activity. The high-oxygen atmosphere induces the formation of protein carbonyls and lipid oxidation products. The use of MAP also caused negative changes in the technological properties and colour parameters of the muscles during storage. From the point of view of physicochemical analysis and texture properties, muscles stored in the DRY-AGED and VAC system showed no visible signs of deterioration and could be stored in the refrigerator for up to 21 days. The storage time had a positive effect on the tenderness of the meat and the colour of the DRY-AGED samples. The results indicate that the recommended methods for storing roe meat for 21 days are VAC and DRY-AGED. Further research should be carried out using other gas indicators for MAP packaging for this species. Due to its very low fat content and high protein content, roe meat is a valuable component of a balanced diet. Compared to other game species, venison meat is also rich in micro- and macronutrients.

