INTRODUCTION
The adoption of plant-based diets, driven by increasing health and environmental concerns associated with meat consumption, has been on the rise globally, including in Indonesia. Plant-based diets are often chosen for the potential benefits of reducing the incidence of chronic diseases and mitigating environmental impacts, such as greenhouse gas emissions and deforestation [Springmann et al., 2018; Willett et al., 2019]. Despite the benefits, plant-based diets face certain nutritional limitations. Specifically, they often lack complete essential amino acids, contain antinutritional factors and toxins, and are typically deficient in vital minerals, such as Fe, Zn, and Ca [Foster et al., 2013; Lynch et al., 2018; Neufingerl & Eilander, 2022].
To address mineral deficiencies in plant-based diets, various vegan products are fortified with essential minerals. Mineral fortification is typically carried out during the product formulation process. However, this fortification can lead to challenges, such as a detrimental effect on the product’s sensory quality, reduced bioavailability of the fortified minerals, and potential adverse interactions with other dietary components [Gibson et al., 2020; Kanamarlapudi & Muddada, 2019; Mannar & Hurrell, 2018]. An alternative approach involves the use of filamentous fungi, which have remarkable ability to bind both essential and non-essential metal ions from their environment and increase the content of specific compounds within their biomass [Fomina & Gadd, 2014; Robinson et al., 2021]. Chitin and other cell wall polysaccharides of filamentous fungi are rich in various metal-binding functional groups such as hydroxyl, carbonyl, amino, acetylamino, and carboxyl groups, which enable them to bind and accumulate metal ions [Ayangbenro & Babalola, 2017]. Previous studies have reported the use of Bacillus subtilis and Saccharomyces cerevisiae as carriers for iron and their application to chocolate fortification [Kanamarlapudi & Muddada, 2019]. Thus, mineral fortification during the cultivation of selected yeast and bacteria may be the superior method.
The most popular commercial fungal-based product is Quorn, produced from Fusarium venenatum. In addition, there are various other species of filamentous fungi deemed viable candidates for protein sources, such as Aspergillus oryzae, Rhizopus delemar, and Neurospora intermedia [Wang et al., 2024]. Another potential filamentous fungus is Rhizopus oligosporus. It has an excellent nutritional profile and has been employed as a tempeh starter for centuries in Indonesia, demonstrating its safety and acceptability for consumers. This fungus is known for its high protein and fiber contents and absence of antinutritional factors [Wikandari et al., 2023]. Several previous studies reported that some Rhizopus sp. have been explored for the ability to remove heavy metals, including cadmium (Cd), arsenic (As), copper (Cu), zinc (Zn), and iron (Fe) [Oladipo et al., 2018; Tahir et al., 2017]. Specifically, R. oligosporus has been reported to bind heavy metals, such as mercury (Hg) and copper (Cu) ions [Ozsoy, 2010; Ozsoy et al., 2008]. Therefore, it is hypothesized that fungi can accumulate, within their biomass, minerals that are present in the growth medium. By enriching the growth medium with essential minerals, the resulting fungal biomass is expected to exhibit enhanced nutritional profiles with increased levels of minerals. Moreover, R. oligosporus can be cultivated on various substrates, including by-products from the food industry, thereby reducing production costs.
Information on the potential binding capacity of fungal biomass to minerals is scarce and the effects of mineral supplementation on fungal growth and the mineral adsorption efficiency of R. oligosporus have not been reported. Excessive metals may have toxic effects on filamentous fungal growth, depending on their metal tolerance [Ahmad et al., 2005]. Shalaby et al. [2023] reported that elevated concentrations of minerals, including silver (Ag), zinc (Zn), and iron (Fe), could inhibit the growth of Aspergillus sp. In addition, some minerals have detrimental effects on the color and taste of the final product, which might affect consumer acceptance. This present study aimed to investigate the effects of mineral addition on the growth and sensory characteristics of the produced filamentous fungal biomass and to determine the optimum supplementation concentration and harvest time. This work highlights the potential of enriching cultivation media with essential minerals as a promising approach for enhancing the quality of filamentous fungal biomass.
MATERIAL AND METHODS
Microorganisms
The filamentous fungus used in this study was the commercial starter Raprima (Toko Hasil Bumi Langgan, DIY, Indonesia) which is produced from Rhizopus microsporus var. oligosporus [Hartanti et al., 2015]. The molecular identification conducted by Yarlina et al. [2023] showed that the fungi were 100% similar to R. microsporus (CBS 631.82). The fungi were kept in the refrigerator at 4°C until use.
Cultivation of filamentous fungi
The cultivation of filamentous fungi was carried out according to a previously reported method [Wikandari et al., 2023]. The filamentous fungi were cultivated using a concentration of 5 g/L glucose as the substrate. Fe, Zn, and Ca were added in the form of FeSO4×7H2O, ZnSO4×7H2O, and CaSO4×2H2O, respectively. The mineral concentrations of the medium were selected to meet the recommended daily intake of minerals per consumption of 100 g of fungal biomass [National Academies of Sciences Engineering and Medicine, 2019]. FeSO4×7H2O was added at concentrations of 1.25, 3.75, and 11.25 mg/100 mL media. ZnSO4×7H2O was added at concentrations of 0.50, 1.75, and 5.25 mg/100 mL media, while CaSO4×2H2O was added at concentrations of 50.0, 87.5, and 125.0 mg/100 mL media. Thus, the media contained 0.25, 0.75, and 2.25 mg elemental Fe/100 mL media; 0.11, 0.38, and 1.14 mg elemental Zn/100 mL media; as well as 11.09, 19.39, and 27.71 mg elemental Ca/100 mL media. Medium without mineral addition was used as the control. The media was sterilized at 121°C for 15 min. One gram of starter was inoculated into a 250-mL Erlenmeyer shake-flask containing 100 mL of the media. The mixture was then incubated at room temperature and an agitation speed of 115 rpm for 48 h. Subsequently, the biomass was harvested and separated from the medium, and then washed with distilled water and pressed to remove excess water. The biomass was stored in a freezer at −18°C until use.
Estimation of mineral adsorption and optimal harvest time of filamentous fungal biomass
To evaluate adsorption of minerals during cultivation, the media and biomass samples were taken at 0, 24, and 48 h of incubation. To determine the optimal harvest time for the highest mineral content in filamentous fungal biomass, the incubation time was prolonged until 72 h. The mineral contents of the media and biomass were determined based on the method by Kalayci & Muhammet [2022] using inductively coupled plasma optical emission spectrometry (Agilent 700 Series ICP-OES, Santa Clara, CA, USA). Approximately 0.5 g of the sample was placed into a polytetrafluoroethylene digestion vessel to which 1.5 mL of HNO3 and 2 mL of H2O2 were added. The sample was kept at room temperature for 6 h. The vessel was then closed for digestion at 170°C for 18 min, then left to cool overnight. The sample was transferred to tubes and diluted to 10 mL with ultrapure water for ICP-OES analysis. The efficiency of mineral adsorption was calculated according to Akpomie et al. [2015] using Equation (1):
where: C0 is the initial mineral concentration in the media (mg/100 mL) and Ce is the final mineral concentration in the media – after 48 h of incubation (mg/100 mL).
Sensory evaluation
The filamentous fungal biomass was served as vegan nuggets for sensory evaluation. The composition of the nuggets included fungal biomass (60%), white bread (6%), tapioca flour (6%), corn starch (1%), modified tapioca (10%), onion (8%), garlic (2%), mushroom seasoning (1%), salt (1%), sugar (3%), white pepper (0.4%), and nutmeg (0.1%), with all percentages calculated by weight. The frozen fungal biomass was first thawed and pressed to remove excess water. The binders and seasonings were minced using a food processor (Phillips HR7627, Amsterdam, The Netherlands) and incorporated with the fungal biomass. The nugget was formed in a tray (40×40 cm2), steamed for 15 min, then coated with batter and breadcrumbs. Afterwards, it was fried (180°C) for 6 min and served to panelists with water and a cracker.
The sensory properties of the fungal nuggets were evaluated by 50 untrained panelists from the Gadjah Mada University, aged between 18–50 years. Individuals with any health problems (e.g., anosmia, ageusia) or known allergic reactions to fungi were excluded. The sensory evaluation was conducted using a 7-point hedonic scale ranging from “extremely dislike” (score = 1) to “extremely like” (score = 7), with panelists assessing attributes including color, taste, and overall liking.
Color analysis
The fungal nuggets (2×2×1.5 cm3) were cut into half to expose the internal color, which was subsequently analyzed using a chromameter (Konica Minolta Co. CR-400, Chiyoda, TYO, Japan). The color coordinated of CIELab space were determined as lightness (L*), redness (a*), and yellowness (b*) [Pathera et al., 2017].
Fourier transform infrared spectroscopy analysis
Fourier transform infrared spectroscopy (FTIR) analysis was carried out to discover the influence of mineral addition on functional groups of fungal biomass compounds. The fungal biomass was dried in the oven at 105°C for 24 h, then finely ground into powder. A total of 1 mg of the dried fungal biomass was measured and characterized using an attenuated total reflectance (ATR) FTIR spectrophotometer (Bruker Vertex80, Billerica, MA, USA) in the wavenumber range of 400–4,000 cm−1 with a scanning number of 32 and resolution of 4 cm−1.
Statistical analysis
The filamentous fungal cultivation was performed in triplicate, and the analyses were conducted in duplicate. The results were presented as mean and standard deviation (SD). One-way analysis of variance (ANOVA) followed by Duncan’s multiple range test (DMRT) was performed for multiple comparisons of the means (p<0.05) using IBM SPSS Statistics version 25.0 (International Business Machines Corporation, Armonk, NY, USA).
RESULTS AND DISCUSSION
Effect of mineral addition on the growth of filamentous fungi
This study focused on investigating the effects of mineral addition on fungal growth and determining the mineral concentrations that do not interfere with fungal growth. Figure 1 shows that the addition of Fe and Zn at different concentrations did not significantly affect the growth of filamentous fungi compared to the control. A previous study reported similar results: the addition of various concentrations of Cd, Cu, lead (Pb), As, and Fe did not affect the growth of R. microsporus [Oladipo et al., 2018]. This study also assessed the tolerance indices of R. microsporus, which exhibited high to very high tolerance in the media enriched with Cd (25–100 mg/kg), Cu (125–1,000 mg/kg), Pb (100–400 mg/kg), As (125–500 mg/kg), and Fe (200–800 mg/kg). Rhizopus species revealed high tolerance to several metals at various concentrations [Ahmad et al., 2005; Sey & Belford, 2021; Zafar et al., 2007]. The metal tolerance of filamentous fungi depends on the isolation site, toxicity, and concentration in the growth media [Zafar et al., 2007].
Figure 1
The effects of iron (A), zinc (B), and calcium (C) addition on the growth of filamentous fungal biomass of Rhizopus oligosporus. Different letters above bars represent significant differences (p<0.05).

However, the addition of Ca at 125 mg/100 mL media resulted in a significant decrease in the growth of filamentous fungi (Figure 1). Calcium is one of the essential nutrients for fungal growth, but it can be toxic and inhibit growth at excess concentration [Ye et al., 2022]. The presence of calcium in appropriate concentration can promote the microbial growth and activity since it acts as an enzyme activator. However, excessive calcium can easily inhibit the microbial metabolism through the modification of cell membranes, thereby blocking the mass transfer between microbes and the substrate [Yang et al., 2022]. Furthermore, Kumar & Dwivedi [2021] reported that the interaction between filamentous fungi and metals can be either positive or negative, depending on the fungi’s tolerance to the metals in the cultivation media. The decrease in the growth rate could be due to metal toxicity, nutritional imbalance in the media, or enzyme inactivation. The present study results demonstrate that Fe, Zn, and Ca can be added in concentrations of 3.75 mg/100 mL, 1.75 mg/100 mL, and 87.5 mg/100 mL, respectively, without interfering with fungal growth.
Adsorption of minerals in filamentous fungi
To further investigate mineral adsorption by fungal biomass, the mineral content was analyzed in the cultivation media and in the fungal biomass. The amount of mineral added to the medium was the optimum mineral concentration obtained from the previous step. The mineral content of the fungal biomass cultivated on the media supplemented with minerals and on the media without supplementation (control) after 48 h of incubation is presented in Figure 2. The mineral content of the fortified-fungal biomass was 5–13 times higher than that of the control. These results indicate that R. oligosporus can bind and accumulate minerals, which is consistent with findings from several previous studies which demonstrated its ability to bind heavy metals, such as Hg and Cu [Ozsoy, 2010; Ozsoy et al., 2008]. Filamentous fungi can bind and accumulate metal ions in two stages. In the first stage of the metal-fungi interaction, the cell wall binds the metal ions through a metabolically passive process. The compounds of cell walls of filamentous fungi are often negatively charged due to the presence of carboxyl and phosphate groups that allow the adsorption of positively charged metal ions through ion exchange, complexation, and physical adsorption. In the second stage, metal ions are transported into the cell through a metabolically active process [Kumar & Dwivedi, 2021; Zabochnicka-Światek & Krzywonos, 2014].
Figure 2
Mineral content of the filamentous fungal biomass of Rhizopus oligosporus with and without (control) mineral supplementation after 48 h of incubation. Different letters above bars, separately for each mineral, represent significant differences (p<0.05).
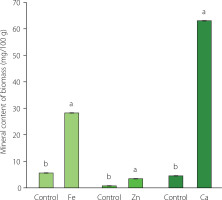
The dietary requirements of Fe, Zn, and Ca are influenced by many factors, such as gender, age, and type of diet. The recommended daily allowance (RDA) of Fe, Zn, and Ca varies between 7–27, 3–13, and 700–1,300 mg/day, respectively [National Academies of Sciences, Engineering, and Medicine, 2019]. In the present study, the contents of Fe, Zn, and Ca in filamentous fungal biomass after 48 h of incubation were 28.26, 3.45, and 63.04 mg/100 g, respectively (Figure 2). This finding suggests that the consumption of 100 g of filamentous fungal biomass could meet the RDA of iron and fulfill the RDA of zinc for children 1–3 years old. However, the calcium content in filamentous fungal biomass would need to be increased to meet the RDA.
The mineral adsorption data for the filamentous fungal biomass are presented in Table 1. The results show that the iron content in the cultivation media decreased during incubation and that the efficiency of Fe adsorption in fungal biomass was 73.57%. This could be due to the presence of iron in the soluble ferrous form, which is taken up more efficiently than the insoluble form. The Fe ions can then be absorbed through a ferrous iron transporter [Philpott, 2006].
Table 1
Mineral content in the media during cultivation of the filamentous fungal biomass of Rhizopus oligosporus and efficiency of mineral adsorption.
The adsorption efficiencies of Zn and Ca, 4.74% and 9.55%, respectively, were lower than that of Fe (Table 1). This suggests that R. oligosporus may have less affinity for Zn and Ca. According to Tahir et al. [2017], the biosorption efficiency of zinc(II) by a group of fungi, including A. oryzae, Penicillium chrysogenum, and Rhizopus oryzae, ranges from 0.5% to 84.4% under various cultivation conditions. However, similar studies regarding the efficiency of calcium adsorption by filamentous fungi are lacking. Furthermore, the efficiency of mineral adsorption by filamentous fungi is associated with several environmental factors, such as pH, temperature, contact time, as well as concentration and age of the biomass [Zabochnicka-Światek & Krzywonos, 2014].
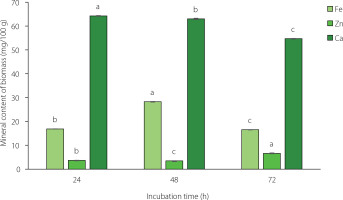
Optimal harvest time of filamentous fungal biomass for a high mineral content
Metal uptake can be influenced by the age of the biomass, which affects the properties of the fungal cell wall and plays a vital role in adsorption mechanisms. To determine the optimal harvest time ensuring a high mineral content of the filamentous fungal biomass, the mineral content after 24, 48, and 72 h of incubation was monitored (Figure 3). The results show that the optimal harvest times for filamentous fungi with Fe, Zn, and Ca supplementation were 48, 72, and 24, respectively. The Fe and Ca contents of the filamentous fungal biomass decreased after 48 h of incubation. Fungal biomass age is one of the determinants of optimal adsorption conditions. In a study on metal biosorption, Shalaby et al. [2023] reported that extending the incubation time resulted in a lower removal efficiency of metal ions by Aspergillus sp. fungus. However, the Zn content of the filamentous fungal biomass increased between 48 and 72 h of incubation (Figure 3). The diverse trends among the minerals might be due to the different adsorption rates of the minerals in the fungal biomass. The mechanisms associated with mineral content changes and incubation time require further investigation.
Sensory scores and color coordinates of fungal nuggets
The product’s sensory properties are the primary driving force behind purchase intention. Several minerals are dark in color, which might affect the color of the final product. Therefore, a sensory evaluation was conducted to investigate the effect of mineral supplementation of filamentous fungal biomass on consumer preferences for the fungal-based nugget product. The results are shown in Figure 4. The nuggets produced from the Ca-enriched fungal biomass received the lowest taste score compared with the control and the other samples. A slightly bitter, sour taste was expressed in the Ca sample. This suggests that the addition of calcium may cause a sensory change in taste, which is in accordance with a previous study reporting that calcium fortification at a concentration of more than 0.3% changed the taste and flavor of beef sausage [Prasetyo & Prayitno, 2021]. Furthermore, Palacios et al. [2021] mentioned that high amounts of calcium have been shown to increase acidity, chalkiness, and bitterness.
Figure 4
Sensory scores of nuggets produced from the mineral-enriched fungal biomass and non-enriched fungal biomass (control).
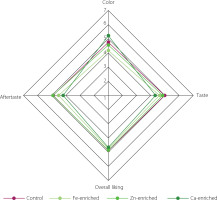
The study results reveal that the color scores given by the panelists to the Zn and Ca samples were not different from those of the control (Figure 4). However, the nuggets produced from the Fe-enriched fungal biomass received lower scores than the control. The color properties, including lightness, redness, and yellowness, of the fungal nuggets were further analyzed using a chromameter. The results show that the Fe sample had the lowest lightness value (Table 2), which suggested that iron might have a detrimental effect on the filamentous fungal nuggets. Iron fortification can cause unacceptable color changes in food [Hurrell, 2021]. Nuggets are expected to be white in color; therefore, the addition of excessive iron, which is a colored substance, might affect the color of the final product and consumer acceptance. Similar results were obtained from the iron fortification of chocolate [Kanamarlapudi & Muddada, 2019], yogurt [Santillán-Urquiza et al., 2017], extruded snack [Bhat et al., 2017], and cheese [Shoukat et al., 2025]. The effects of Fe supplementation may be due to the characteristics of ferrous sulfate, which is water-soluble and has the potential to cause discoloration in food. The presence of Fe may also accelerate the Maillard reaction during heating, which may result in darker and more intense color [Kathuria et al., 2023]. Various novel fortification techniques were studied to mitigate the discoloration, such as encapsulation and chelation. However, these techniques may affect the production cost and reduce the nutrient bioavailability [Manzoor et al., 2024].
Table 2
Color coordinates of nuggets produced from the mineral-enriched fungal biomass and non-enriched fungal biomass (control).
| Fungal nugget | L* | a* | b* |
|---|---|---|---|
| Control | 49.36±0.58b | −0.84±0.04a | 13.31±0.27a |
| Fe-enriched | 48.23±0.52c | −0.76±0.08a | 13.09±0.11a |
| Zn-enriched | 51.00±0.62a | −0.91±0.21ab | 13.57±0.40a |
| Ca-enriched | 51.42±0.06a | −1.16±0.15b | 13.52±0.23a |
The addition of a single mineral did not affect significantly the overall liking of fungal nuggets (Figure 4). This indicates that the sensory characteristics of the fungal nuggets were still acceptable to consumers. Kanamarlapudi & Muddada [2019] conducted a study on iron fortification in chocolate using biosorbed biomass containing B. subtilis and S. cerevisiae and obtained similar results that the fortification with biosorbed biomass did not lead to any significant sensory changes.
Results of Fourier transform infrared spectroscopy analysis
Different studies reported the interaction of various ions with biomass such as Arthrospira platensis and Chlorella vulgaris [Ferreira et al., 2011], Padina tetrastromatica [D’Souza et al., 2008], Trichoderma harzianum [Shoaib et al., 2013], R. oligosporus [Ozsoy et al., 2008]. Overall, they indicated that the uptake of metal ions from the medium affected the carboxyl, carbonyl, phosphate or, amide groups. The absorption bands in the 3,700 to 3,200 cm⁻1 range are usually attributed to O–H and N–H stretching. The control and fungal biomass supplemented with Ca and Fe had a major peak at 3,276 cm−1, with Ca having the lowest intensity (Figure 5). However, in the sample supplemented with Zn, the broad band appeared at a higher wavelength (3,285 cm−1) and showed a significant increase in intensity. This could suggest that Zn2+ interfered with the hydrogen bonding network of hydroxyl and amine groups, which might have been due to the direct coordination between Zn2+ and biomolecular functional groups, leading to weaker hydrogen bonds and the NH group. A similar phenomenon was observed when P. tetrastromatica was grown in the medium with Cd which transformed the OH and NH groups from non-bounded to bounded ones [D’Souza et al., 2008].
Figure 5
Fourier transform infrared spectra of the filamentous fungal biomass of Rhizopus oligosporus with mineral supplementation and without supplementation (control).
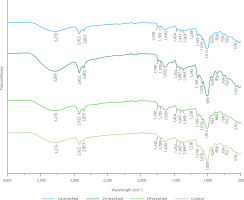
In general, changes in spectra intensity at 800–1,800 cm−1 could indicate changes in the protein and polysaccharide composition of the biomass in response to the presence of metal ions in the growth medium [D’Souza et al., 2008]. The band at 999–1,012 cm−1 could indicate the C–C and C–O stretching of the carbohydrate fraction [Ferreira et al., 2011]. However, Majumdar et al. [2008] mentioned that the bands in the region of 1,078–995 cm−1 were due to the presence of the phosphate group in the biomass. Therefore, in our spectra, it was possible that this peak represented an overlap of those functional groups, as indicated by the visible shoulders on the band (Figure 5). Therefore, the changes in peak indicated that the presence of metal ions affected not only the carbohydrate composition but also the phosphate group of the biomass. Among all samples, the Zn-treated biomass exhibited the most noticeable spectral changes. In addition, peak positions at 1,407 cm−1 can be attributed to the COO− group present in the biomass [Majumdar et al., 2008]. The change in the peak wavelength and intensity of the fungal biomasses (Figure 5) seemed to be caused by the binding of the metal ions. The amide I peak was observed at 1,630 cm−1 in the control and Ca-fortified sample and shifted to slightly lower and higher values in the sample with Fe and Zn, respectively. In addition, changes in the intensity were also observed, highlighting the effect of metal ions on the biomass protein content and properties. In this study, a band was observed at approximately 1,740 cm−1, corresponding to the C=O stretching vibration of ester carbonyl groups. The presence of this peak in our spectra indicated the presence of lipid-related ester groups in the fungal biomass [D’Souza et al., 2008]. The slight intensity change of this band across treatments may also suggest interactions with metal ions.
Kheeree et al. [2022] and Wang et al. [2018] mentioned that metal ions can form bonds with carboxyl, carbonyl, phosphate or amide groups, which is in line with our observations. Interaction between metal ions (positively charged) and functional groups (negatively charged) in the biomass could form new complexes. The result of the FTIR measurement confirmed the formation of new complexes between metal ions and functional groups in the fungal biomass. This is in accordance with the results of mineral analysis showing an increase in mineral content of the fungal biomass supplemented with essential minerals.
CONCLUSIONS
This study showed that R. oligosporus could adsorb essential metals, including Fe, Zn, and Ca, with optimal harvest times of 48, 72, and 24 h, respectively. The addition of minerals at concentrations of 3.75, 1.75, and 87.5 mg/100 mL for Fe, Zn, and Ca, respectively, did not affect the fungal growth in comparison with the control. It also did not affect the overall liking and taste of nuggets made from the mineral-enriched fungal biomass. This study revealed that the supplementation of the cultivation media with minerals offered a potential natural fortification method for the production of high-mineral vegan products. Future studies may focus on the evaluation of mineral bioavailability in filamentous fungal biomass.