ABBREVIATIONS
DL, drying loss; HH, hypocholesterolemic to hypercholesterolemic fatty acid ratio; IA, index of atherogenicity; IT, index of throm-bogenicity; MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acids; SFA, saturated fatty acids; SL, smoking loss.
INTRODUCTION
Fish represents a vital source of nutrients essential for maintaining human health. Numerous scientific studies highlight the beneficial impact of fish consumption on various aspects of human well-being, particularly in cardiovascular disease prevention [Milićević et al., 2022; Shalini et al., 2021]. Among the range of fish products, smoked fish holds a prominent position due to its traditional appeal and widespread popularity. The quality of raw sprats is the primary factor affecting the quality of the smoked product. However, it is well-established that the quality of fish, particularly within the fishing season, is subject to fluctuations influenced not only by the inherent quality of the raw material but also by factors such as the method of catch and storage conditions preceding further processing [Sabu & Sasidharan, 2020].
Typically, in the Baltic Sea, the sprat fishing season starts in September or October and ends in April or May. Fish flesh quality parameters are influenced by seasonality, sex, age, size, skin properties, and geographical location, all contributing to changes in its lipids and proteins [Abbas et al., 2008]. In the spring, the Baltic sprat lipid content is low, but in the autumn (October, November), it reaches its highest level. The lipid and moisture contents influence texture, while protein content remains stable throughout the fishing season, although amino acid composition varies [Usydus et al., 2012].
To ensure a year-round supply of raw material, sprats are frozen, but it is essential to maintain the stability of the nutritional composition in fish during freezing and storage. Freezing is a preservation method that ensures longer shelf-life of fish but causes structural damage to muscle cells, leading to changes in meat quality. Before use, frozen fish needs to be thawed, which alters its sensory attributes and nutritional value [Trigo et al., 2018]. Proper thawing aims to minimize quality changes, but impacts texture, moisture loss, and microbiological activity [Yang et al., 2019]. The pH of fresh fish or thawed fish is close to neutral. Babikova et al. [2020] reported a positive correlation between pH, moisture content, and textural properties. The pH is also responsible for changes in fish color. The main physical changes which occur in fish upon processing are those observed in texture and color, which are the main factors influencing consumer acceptance [Abraha et al., 2018].
While extensive literature exists regarding Atlantic fish and their processing techniques [Dawson et al., 2018; Fernandes et al., 2024; Jiang et al., 2024], including smoking and quality enhancement methods [Güngören et al., 2023; Liu et al., 2023; Sutikno et al., 2019] and similar research on sardines and their practical applications [Scheuer et al., 2024; Serdaroglu et al., 2015], there is a dearth of data concerning Baltic sprats – a staple in many parts of Europe – and the seasonal variations in their quality. Sparse are also investigations on applying prior processing techniques to fresh and frozen fish for improvement of the production yield by mitigating smoking losses and minimizing drying in storage chambers while enhancing quality indicators such as texture, color, pH, sensory attributes, and microbiological safety.
In light of these gaps in knowledge, this study aimed to evaluate the chemical composition of Baltic sprats throughout the fishing season, while examining the texture and color of smoked sprats produced thereafter. Smoked sprats were produced from fresh fish and fish from the same batches after one year of frozen storage and thawing.
MATERIALS AND METHODS
Fish collection and processing
Fresh Baltic Sea sprats caught during the fishing season from November to March 2020 in the Baltic Sea FAO27, III.d.28.2 using a trawl were used in the present study. The fresh fish were transported to the producing company within 48 h, and the internal temperature of the fish was maintained at 2±2°C. The fish belonged to freshness category A and size category 1 for sprats described in Council Regulation (EC) No. 2406/96 [EC Regulation, 1996]. Sprats were stored until processing in a container covered with ice (in a proportion of fish to ice of 1:3) at 2±2°C in a refrigerated room. Fish from the same batches were frozen and packed in plastic bags, then placed inside cardboard boxes. To produce frozen sprats, fresh sprats were sorted by size and placed in polyethylene bags (with thickness not exceeding 0.15 mm) in 10-kg portions. These bags were placed on horizontal frames and quickly frozen at temperatures between –35°C and –39°C for at least 3 to 5 h using a tunnel freezer with periodic operation. The frozen sprats were then stored for one year at –18±2°C with a relative humidity above 85%.
Frozen sprats, packaged in blocks within plastic bags, were thawed in a defrost chamber using water spray and hot water steam until their internal temperature reached 2±2°C, a process took 1 to 2 h). Both fresh and frozen sprats were smoked in a batch-type hot smoking chamber (Reich Foodsystems, Urbach, Germany) using beech wood chips (with moisture below 15%, wood chip size 5–8 mm). Three independent batches of 300–350 g were prepared per sample type. The main parameters for the hot smoking process were 35–40 min of drying at 45 to 60°C, 10–12 min of cooking at 60 to 75°C, 2–5 min of steaming at 75 to 77°C, 10–15 min of adding smoke at 77 to 80°C and cooling to 10°C to facilitate cutting of heads or tails after smoking. All smoked fish samples were analyzed one day after smoking.
Analysis of fresh sprats
Moisture analysis of sprats was conducted following the method of International Organization for Standardization [ISO 1442:1997]. Briefly, a 3-g homogenized sample of fresh sprat carcass (without the head, blended into a paste) was mixed with 9 g of pre-heated sand. The mixture was dried at 150°C for 60 min until a constant weight was reached. After drying, the sample was placed in a desiccator to cool and then weighed.
The sprat pH analysis was performed using a pH meter (Jenway 3520, EU). To measure pH, a 1-g homogenized fish sample was mixed with 9 g of distilled water and immediately tested for pH.
The protein content was determined in triplicate using the Kjeldahl method [ISO 5983-2:2009], with 2 g of the sample analyzed on a Kjeltec 2300 automatic analyzer (Foss Analytical, Höganäs, Sweden). The protein content of sprats (g/100 g) was calculated using a nitrogen conversion factor of 6.25.
The lipid content of the raw fish was measured in triplicate via Soxhlet extraction using an Soxtec Avanti 2050 instrument (Foss Analytical, Höganäs, Sweden) and petroleum ether, following a standard procedure [ISO 1443:1973].
Amino acid and fatty acid profiles were analyzed in the accredited laboratory J. S. Hamilton Sp. z.o.o. (Gdynia, Poland). All results were expressed as g/100 g of product. The fatty acid profile was determined using gas chromatography after fatty acid transmethylation under alkaline conditions according to standard procedures [ISO 12966-1:2015-01; ISO 12966-2:2017-05; ISO 12966-4:2015-07].
The ratio between the saturated fatty acids (SFA) and the unsaturated fatty acids (UFA) was demonstrated by the index of atherogenicity (IA). This index was initially established in 1991 by Ulbricht & Southgate [1991], and can be calculated from Equation (1):
where: MUFA means the sum of monounsaturated fatty acids, PUFAn6 is the sum of n6 polyunsaturated fatty acids and PUFAn3 is the sum of n3 polyunsaturated fatty acids.The index of thrombogenicity (IT) represents the ratio between pro-thrombogenic (SFA) and anti-thrombogenic fatty acids (MUFA, PUFAn6, and PUFAn3), and was calculated using Equation (2):
The hypocholesterolemic to hypercholesterolemic fatty acid ratio (HH index), as detailed in the study by Chen & Liu [2020], interprets the dynamics of cholesterol metabolism and was calculated using Equation (3):
Proteinogenic amino acid profile was analyzed as outlined in [PB-53/HPLC ed. II of 30.12.2008], where aspartic acid was the sum of asparagine, aspartic acid and its salts; glutamic acid was the sum of glutamine, glutamic acid and its salts; cysteine was the sum of cystine and cysteine.
Analysis of smoked sprats
Moisture content and pH of smoked sprats were evaluated according to the same methods as applied to fresh sprats.
The smoking losses (SL) were calculated using weight (W) in g before and after smoking, as shown in Equation (4):
The drying losses (DL) are observed during smoked sprat cooling and storage. Therefore, for the study purposes, fish after smoking was stored in perforated plastic boxes at 2±1°C for cooling. The DL was determined 24 h after smoking and calculated according to the Equation (5):
The texture analysis was conducted using a Texture Analyzer TX. HD Plus, equipped with the Warner-Bratzler knife blade set (Stable Microsystems, Godalming, UK). Before analysis, each smoked fish specimen was cut to remove the bone, resulting in two separate pieces of smoked sprat fillet. The width of each fillet was measured before cutting and entered into the software. Subsequently, each fillet was placed longitudinally on the slotted platform of the texture analyzer. The blade was then set to move at a speed of 2 mm/s for a distance of 10 mm. This process yielded the cutting strength (measured in N/mm), indicating the firmness of the fish. Seven measurements were performed for each sample.
The color assessment was conducted using a ColorTec-PCM color meter (Accuracy Microsensors Inc., Vernon Hills, IL, USA) equipped with Color Tec-Color Soft QCW software. Color measurements were taken on the surface of 5 to 7 smoked fish specimens, with no more than two different locations measured on each fish side. A total of 10 measurements were obtained, capturing variations across the samples. In the CIEL*a*b* system, the value L* represents lightness on a scale from 0 to 100, ranging from black to white, while the value a* indicates redness (+) or greenness (–), and the value b* reflects yellowness (+) or blueness (–).
Statistical analysis
Samples underwent triplicate testing, except for color and textural parameters, which were analyzed with at least 7 repetitions. Means and standard deviations were calculated using MS Office Excel 2016 (Microsoft, Redmond, WA, USA) software. Analysis of variance (ANOVA) and t-tests were conducted using XLSTAT 2020 (Addinsoft, New York, USA). Comparisons were made at a significance level of p≤0.05. To investigate the relationship between chemical compositions and fishing periods, correspondence analysis (CA) was employed. Differences in categorical variables were analyzed using the t-test (p≤0.05).
RESULTS AND DISCUSSION
Composition of fresh Baltic sprats depending on the season
The nutritional composition of sprats varied significantly across individual periods of the fishing season (Table 1). This study revealed a high moisture content in the sprats caught during the spring, which was consistent with findings from other studies [Timberg et al., 2011; Usydus et al., 2012], while the highest lipid content was found in the sprats from the autumn catch when the moisture content was the lowest. Results showed that the protein content remained stable throughout the season. According to Rasul et al. [2021] the variations in fish composition are influenced by environmental factors such as water temperature, pH, salinity, and food availability during the season. The moisture content varies due to osmoregulation during the migration process. Regarding lipids, it is known that fish utilize those as an energy source during certain periods. Additionally, changes in fish composition are also affected by factors such as fish species, age, size, sex, habitat, and breeding season [Chen & Liu, 2020; Jiang et al., 2024; Usydus et al., 2012].
Table 1
Fresh sprat composition across the fishing season.
| Variable | Autumn | Winter | Spring |
|---|---|---|---|
| Protein content (g/100 g) | 16.4±1.3a | 14.9±1.2a | 16.8±1.3a |
| Lipid content (g/100 g) | 19.2±1.7a | 12.9±1.1b | 12.1±1.1b |
| Moisture (g/100 g) | 62.4±1.2b | 69.2±1.2a | 70.0±1.2a |
Sprats were recognized as a source of proteins with valuable amino acid profile. The highest contents among essential amino acids were found for lysine and leucine (Table 2). During the fishing seasons, noteworthy changes occurred in content of histidine, which decreased from 0.47 g/100 g in the autumn to 0.34 g/100 g in the spring. The highest contents were observed for non-essential amino acids such as aspartic acid and glutamic acid, which is consistent with the findings reported by Vázquez et al. [2023]. Sprats contain a significant amount of red muscle, which correlates with a higher content of free amino acids [Bodin et al., 2022]. This is influenced by pH, which in fresh sprats is higher in the winter, resulting in reduced decarboxylation of certain amino acids [Jääskeläinen et al., 2023].
Table 2
Amino acid content of sprats across the fishing season (g/100 g of product).
Throughout the fishing season, there was a notable reduction in fatty acid content, particularly in n3 fatty acids, which exhibited a threefold decrease (Table 3, Table S1 in Supplementary Materials). Specifically, total n3 fatty acids decreased from 6.3 g/100 g in the autumn to 1.9 g/100 g in the spring. As highlighted by Merdzhanova et al. [2018], sprats, being oily fish, are naturally rich in n3 fatty acids. The highest levels of n3 fatty acids were determined in the sprats caught in the autumn, indicating their elevated nutritional value during this period. This seasonal variation can be attributed to the weakening of protein-lipid linkages and reduction in lipid autoxidation as season progresses, as suggested by Beltrán & Moral [1991].
Table 3
Fatty acid groups in sprats across the fishing season (g/100 g of product).
The primary fatty acids in sprats predominantly consist of n3 fatty acids, a finding consistent with previous research [Scheuer et al., 2024]. In autumn, the fatty acid composition follows the pattern PUFA > MUFA >SFA. By spring, this pattern shifts, with MUFA becoming dominant followed by SFA and PUFA. This seasonal variation differed from the findings reported by Merdzhanova et al. [2018], but may be explained by changes in the sprat diet during spring, particularly a lower intake of lipids and a higher MUFA content. This suggests a reduced presence of cold-water copepods, which are typically part of the sprat diet in colder months. The seasonal changes in fatty acid composition were further confirmed by Jiang et al. [2024], reinforcing the idea that diet and environmental factors significantly influence the nutritional profile of sprats throughout the fishing season.
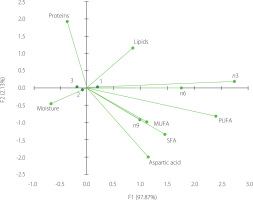
The plot diagram (Figure 1) illustrating the association between the analyzed periods of the fishing season and chemical composition shows that 97.87% of the variability was attributed to factor 1 (F1), while 2.13% was attributed to factor 2 (F2). Moisture and n3 fatty acid contents were the most important contributors to F1, each with a contribution of 0.252. In turn, protein content was the most significant contributor to F2, with a contribution of 0.522. The correspondence analysis indicates a linear relationship between moisture and lipid contents. Winter and spring fish were more similar in their chemical composition profiles, whereas the autumn fish showed more pronounced differences.
Figure 1
Correspondence analysis (CA) plot using chemical compositions and periods across the fishing season, including autumn (1), winter (2), and spring (3).
The nutritional value of fish is often assessed based on the PUFA/SFA ratio, which demonstrates the balance between polyunsaturated and saturated fatty acids. For sprats, this ratio ranged from 1.42 to 0.95 towards the end of the fishing season, while for other fish species, it varies from 0.5 to 1.62 [Chen & Liu, 2020]. The IA, which indicates the relationship between saturated and unsaturated fatty acids, increased from 0.43 to 0.66 during the whole fishing season (Table 4). Its lower value suggests a healthier lipid profile, implicated in a reduced risk of heart disease development. In turn, the IT of the sprats ranged from 0.14 to 0.21. This index, reflects the ratio between saturated fatty acids and the combined amount of monounsaturated fatty acids, n6, and n3 fatty acids. Lower IT values are associated with a reduced risk of cardiovascular problems due to reduced proportion of saturated fatty acids, which may increase the risk of clot formation. Łuczyńska et al. [2017] determined its values at 0.70 for herring and 0.31 for carp, with a range of 0.14 to 0.87 for most fish species. Furthermore, the HH index, which characterizes cholesterol metabolism, fluctuated throughout the fishing season (Table 4). Its higher value indicates a lipid profile that is more beneficial for lowering cholesterol levels, thus promoting better cardiovascular health.
Table 4
Nutritional indices of sprats across the fishing season.
The lowest values of atherogenicity and thrombogenicity indices were recorded in the autumn sprats, while the HH index was the highest in this season, indicating a higher nutritional value of the sprats. According to Chen & Liu [2020], IA and IT are the most commonly used to assess the composition of fatty acids. However, while these indices can be useful for comparing foods, no specific recommended values have been developed.
Physiochemical characteristics of smoked sprats
Similar to fresh fish, the significantly higher moisture content was determined in smoked sprats produced from the fish caught during the spring season and the highest lipid content in those from the autumn catch fish, when the moisture content was the lowest (Table 5). However, smoked sprats produced from frozen fish did not exhibit the same pattern. Reduced moisture content was observed in the smoked sprats produced from frozen winter and especially frozen spring fish compared to those produced from fresh fish. This difference may be related to the higher moisture content and distinctive water distribution patterns in raw fish tissue [Loje et al., 2007], coupled with increased tissue damage induced by ice crystals [Martinez et al., 2012; Ruiz-Alonso et al., 2021]. Our study results indicate that a higher moisture loss occurred during freezing, subsequent frozen storage, and thawing in the sprat samples with a higher initial moisture content. This resulted in lower smoking and drying losses in these samples compared to their fresh counterparts (Table 5).
Table 5
The pH of raw sprats and physicochemical characteristics of smoked sprats across the fishing season.
The pH of sprats was affected by both frozen storage and smoking. Following one year of frozen storage, a decrease in pH was noted, with the most significant decline observed in the sprats caught in the spring season (Table 5). Conversely, smoking led to an increase in the pH of the sprats compared to that of raw fish. The impact of analyzed periods of the fishing season was evident in the moisture content of the smoked fish, as well as in smoking and drying losses. In production, smoking losses typically range from 35% to 50%. The results below 30% are considered excellent, while losses exceeding 50% are deemed unacceptable.
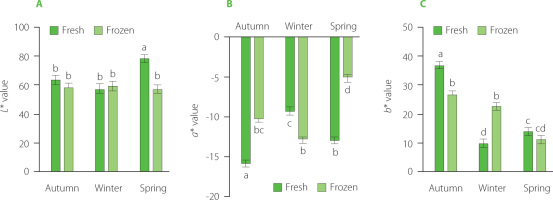
The color values L*, a*, and b* determined for the smoked sprats changed throughout the fishing season, indicating differences in the product made from fresh or frozen fish (Figure 2). When comparing smoked sprats produced from fresh vs. frozen sources, distinct differences in redness (a*) and yellowness (b*) were evident. The color of the fish changes during heat treatment, primarily influenced by the non-enzymatic Maillard reaction [Liu et al., 2022]. During heat treatment, protein reduces the porosity in the product’s structure and increases opacity, thereby enhancing light reflection. It is well known that opacity increases when myosin and actin denature [Valentim et al., 2024]. Smoking significantly decreases lightness and increases yellowness but has less impact on redness [Astruc et al., 2022]. The yellowness and redness were observed to be lower in smoked spring fish, likely due to the lower lipid content in raw fish, which may correlate with a reduced concentration of fat-soluble pigments [Nie et al., 2011]. Significantly lower redness was also found for Atlantic mackerel when comparing fresh and frozen fillets [Fernandes et al., 2024]. When comparing fresh and frozen sprats, the color changes as the sprats are frozen and during storage, pigments from fish surface are released from tissues and begin oxidation, causing the sprat tissue to darken and fade.
Figure 2
Color values determined for smoked sprats produced from fresh or frozen sprats: (A) lightness value, L*; (B) redness value, a*; (C) yellowness value, b*. Bars show average value (n=7). Error bars represent standard deviation. Different letters above/below bars show significant differences p≤0.05 (t-test).
The smoked sprats produced from fresh sprats caught in the autumn exhibited higher hardness, measuring 0.99 N/mm, in contrast to those produced from fresh spring catch fish, for which 0.49 N/mm was recorded (Figure 3). Throughout the fishing season, texture parameters decreased by at least two times. Likewise, smoked sprats manufactured from frozen sprats demonstrated a decrease in hardness across the seasons. At the beginning of the fishing season, the hardness was at 0.77 N/mm, whereas by the fishing season’s end, it decreased to 0.36 N/mm.
Figure 3
Texture of smoked sprats produced from fresh or frozen sprats. Bars show average value (n=10). Error bars represent standard deviation. Different letters above bars show significant differences at p≤0.05 (t-test).
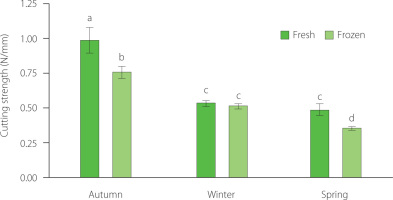
Our study results suggest that smoked sprats produced from the fresh autumn fish exhibited the highest texture values. Texture characteristics may be related to moisture and lipid content; as excessive lipid content can lead to decreased muscle strength [Jiang et al., 2024]. Changes in hardness can result from muscle shear resistance, protein extraction capacity, solubility, and viscosity changes during frozen storage [Xie et al., 2023]. Conversely, the reduced hardness observed in smoked sprats manufactured from the spring catch may be attributed to the higher moisture content in raw sprats. This increase in moisture could lead to a decrease in water-holding capacity due to protein denaturation and aggregation caused by ice crystals [Martinez et al., 2010]. To address this issue, various strategies can be employed to improve the water-holding capacity, such as salting and brining before smoking, which should be further investigated.
CONCLUSIONS
Baltic sprats are nutrient-rich fish, consistently high in protein throughout the fishing season. However, their lipid content undergoes significant changes over the season, significantly decreasing from autumn to spring. Autumn-caught sprats showed the highest n3 levels and the most favorable lipid profile, suggesting greater nutritional value during this period. In spring, the moisture content increased, which affected the physical characteristics of smoked sprats, including texture, color, and pH. The reduced hardness, along with reduced redness and yellowness in smoked sprats produced from the fish caught in the spring was likely a result of the increased moisture content. To ensure consistent quality in smoked sprats year-round, future research should focus on strategies to enhance the quality of spring catches.

