INTRODUCTION
Beetroots are a rich source of main nutrients (especially protein and carbohydrates) with a relatively low energy value (43 kcal/100 g of fresh product). A 100-g portion of fresh beetroot contains about 86.3 g water, 0.2 g fat, 1.0 g protein, 8.4 g carbohydrates, 2.6 g fiber, and 1.5 g ash [Abdo et al., 2020]. In addition, it contains large amounts of biologically active compounds and micronutrients, such as betalains, carotenoids, phenolics, and B-group vitamins (B1, B2, B3, B6, and B9), as well as inorganic nitrates [Chhikara et al., 2019]. Phenolics, carotenoids, and vitamins in beetroots determine their antioxidant, anti-inflammatory, anticancer, antidepressant, and liver-protecting effects [Slavov et al., 2013], as well as antidiabetic, anti-obesity, antimicrobial, antihypertensive and cognitive improvement properties [Hadipour et al., 2020]. Natural pigments found in beetroots, such as betanins, are used to enhance the red color of various food products, e.g., ice cream, jams, jellies, tomato pastes, sauces, drinks, sweets, and desserts [Punia Bangar et al., 2023]. To take advantage of the health-promoting properties of beetroot, they can be eaten raw, fermented, cooked, baked, or dried as an addition to various types of dishes and salads, but also in the form of juice or beetroot leaven [Szekely & Mate, 2022].
Recently, fermented products have become increasingly popular. This is related to new nutritional trends and growing consumer awareness of food processing [Janiszewska-Turak et al., 2022]. Beetroot leaven (also called beetroot kvass) is, next to fermented beets, the main product of the beetroot lactic fermentation process and is an element of Polish cultural heritage [Staninska-Pięta et al., 2023]. According to traditional Polish recipes, beet pickling (beetroot fermentation) involves placing fresh beets (and spices, e.g., garlic, pepper, allspice or bay leaves) in glass (clay, stoneware) jars and pouring brine over them. The mixture is then stored in a dark, warm place for about 7–14 days. After this time, both the fermented beets and beetroot leaven (solution) are consumed. Fermentation is considered a simple and valuable biotechnological process to maintain and/or enhance the safety, nutritional, sensory, and shelf-life properties of vegetables and fruits [Di Cagno et al., 2013]. Lactic acid bacteria convert carbohydrates contained in vegetables and fruits mainly into lactic acid, which lowers the pH of fermented products to about 4.0, thus ensuring the microbiological stability of the product by inhibiting the development of unfavorable and pathogenic bacteria [Montet et al., 2014]. Lactic acid bacteria are often considered probiotics beneficial to human health. They improve the microbiological homeostasis in the human intestines, inhibit the growth of pathogens such as Escherichia coli, Salmonella, and Staphylococcus [Zommiti et al., 2020], stimulate the immune system [Latif et al., 2023], or break down some enterotoxins in food [Petrova et al., 2022].
Beetroot leaven is an example of a fermented food that is associated with high nutritive and taste values as well as salubrious effects [Walkowiak-Tomczak & Zielińska, 2006]. It can be a source of valuable probiotic bacteria, and beetroot juice subjected to lactic acid fermentation has been characterized by even stronger antioxidant properties than fresh juice [Jakubczyk et al., 2024]. Despite the high health-promoting value of beetroot leaven consumption, there are few studies determining the impact of individual process parameters on the physicochemical properties of the leaven. An important aspect of the natural fermentation of vegetables is maintaining the appropriate process conditions. For this purpose, the addition of salt is used, the temperature is controlled, and the time of the process needs to be investigated [Janiszewska-Turak et al., 2022]. So far, for example, the effect of boiling on the betalain profiles and antioxidant capacities of red beetroot products has been studied [Sawicki & Wiczkowski, 2018]. In this work, boiling was used as a pre-treatment before the fermentation process, while the fermentation itself was carried out in constant conditions at 23°C, 2–3 mm slices of beetroot and for a maximum period of 14 days. In the cited study, boiling and fermentation reduced the content of betalains by 51–61% and 61–88%, respectively. In another work, the effect of spontaneous fermentation of beetroot against three commercially available starters (Sacco System by Sacco S.r.l., Cadorago, Italy; L. mesenteroides; Sacco System, Italy; Kefir; “Kefir d’acqua” Bionova S.r.l., Villanova sull’Arda, Italy) was compared in relation to volatilomes (fermentation was conducted at 22°C for 19 days) [Casciano et al., 2022]. The cited authors stated that spontaneous fermentation was mostly described by molecules with positive aromatic notes, such as 2-undecanone and 2-nonanone. Fermented beetroot juice or beet leaven were used as a raw material in the spray drying process to produce functional powders [Janiszewska-Turak et al., 2022]. These powders can be used as an additive to food production or as an independent dietary supplement [Janiszewska, 2014].
So far, no standardized production recipes have been developed that would allow for controlled spontaneous fermentation of beetroot slices to obtain leaven of the desired quality. Knowledge about optimizing the lactic acid fermentation process of beetroot in terms of the physicochemical properties of the leaven may also be useful in the subsequent use of fermentation products. Therefore, the aim of this study was to determine the optimal thickness of beetroot slices, temperature and time of fermentation for the physicochemical properties of beet leaven, such as titratable acidity, pH, turbidity, total soluble solid content, color parameters, and contents of total carbohydrates, total phenolics, total betacyanins and total betaxanthins.
MATERIAL AND METHODS
Material
Fresh, red beetroots were obtained from a local supermarket (Olsztyn, Poland). The initial moisture content of fresh beetroots was estimated at 6.29±0.60 kg H2O/kg dry matter (DM) by drying the material in a vacuum dryer (DZ ZBC II, Chemland, Stargard Szczeciński, Poland) at 70°C and 13.3 kPa for 24 h. The ripe vegetables were free from diseases and comparable in freshness and size. Before the fermentation process, they were cleaned with tap water and cut with a vegetable cutter (V-type, HENDI, Robakowo, Poland) into 2-, 4-, and 6-mm slices.
Chemicals and reagents
Analytical-grade reagents such as concentrated sulfuric acid and Folin–Ciocalteau reagent were purchased from Sigma-Aldrich (Saint Louis, MO, USA), and others including phenol, phosphate buffer, sodium carbonate, sodium chloride, and sodium hydroxide from POCH (Gliwice, Poland). Analytical standards such as lactic acid, gallic acid, betanin, vulgaxanthin-I, and glucose were purchased from Sigma-Aldrich, and tocopherols from Calbiochem (Nottingham, United Kingdom). Deionized water was obtained from an HLP 5 deionizer (Hydrolab, Gdańsk, Poland).
Lactic acid fermentation
Each type of beetroot slice (650 g) and spices such as peppercorns (0.5 g), allspice (1 g), bay leaves (1 g), and fresh garlic (15 g) were placed in glass jars of 1.6 L volume. Then, the contents of the jars were poured with a 1.8% sodium chloride water solution. The material-to-solution ratio was 1:1.2 (w/v). The jars were then tightly capped and placed in a dark place for immediate fermentation. The fermentation continued at 15, 20, and 25°C in a laboratory incubator (Q-Cell00/40, Pol-Lab, Wilkowice, Poland) for a maximum of 15 days. During the fermentation, the contents of the jars were mixed once a day, and the leaven samples (100 mL) were taken, then filtered, and frozen at –18°C (GT 4932 Comfort, Liebherr, Bischofshofen, Austria) for the assessment of their quality. The physicochemical properties of the leaven were determined daily for the first seven days of fermentation and then every two days until the 15th day of fermentation. None of the collected samples had any extrinsic tastes or odors and did not show any other irregularities in the organoleptic assessment. Fermentation experiments (per one variant, i.e., for a specific thickness of beetroot slices, temperature and fermentation time) were conducted in separate jars and performed in duplicate (in two parallel jars).
Determination of total soluble solid content and turbidity
The content of total soluble solids (TSS) of leaven was measured at 20°C using the refractometer (type G, Carl Zeiss Jena, Germany), and expressed in Brix % (1 g of dissolved solids in 100 g of solution). The turbidity (TR) was determined using a turbidimeter (TB 211 IR, Lovibond Tintometer, Sarasota, FL, USA), and expressed in nephelometric turbidity units (NTU). Both measurements were performed in triplicate.
Determination of pH and titratable acidity
The pH of leaven was measured using a pH meter (CP-451, Elmetron, Zabrze, Poland). In turn, titratable acidity (TA) was determined according to Abdo et al. [2020] by titration with a 0.2 M sodium hydroxide solution to pH 8.2. Results of titratable acidity determination were calculated based on an equation for a lactic acid curve and expressed as g lactic acid equivalent (LA) per 100 mL of leaven. Both measurements were performed at least in duplicate.
Determination of total carbohydrate content
Total carbohydrate content (TC) was determined according to the phenol-sulfuric acid method described by Zhang et al. [2020]. Firstly, 500 μL of the leavens were mixed in the probe with 200 μL of a 5% aqueous solution of redistilled phenol. Next, 1,250 μL of concentrated sulfuric acid were added. The contents of the test tubes were thoroughly mixed, and then the tubes were placed in a boiling water bath for 20 min. Afterwards, they were cooled in an ice bath for 2 min and left for 15 min at room temperature. Absorbance was measured at 490 nm and recalculated to the standard curve prepared for glucose in the range of 1−100 μg/mL. The total carbohydrate content was expressed in g/100 mL. The analyses were performed at least in duplicate.
Determination of color parameters
Color coordinates of leavens were measured using a spectrophotometer (3Color 9000Neo, TRI-COLOR, Narama, Poland) in a reflection mode under standard D65 illumination, a 10° observer, and an 18 mm diaphragm. The color was expressed in the CIEL*a*b* space, where L*, a*, and b* represented lightness, (+)redness/(−)greenness, and (+)yellowness/(−)blueness, respectively. The total color difference (ΔE) was calculated according to the equations (1-4) [Zielinska & Markowski, 2012]. The color results were averaged from six measurements.
where the reference values of color coordinates were measured for pure brine before fermentation (day 0), and the sample values were measured for beetroot leavens fermented at specified process parameters.Determination of total phenolic content
Total phenolic content (TPC) of leavens was determined according to the assay with Folin-Ciocalteu (FC) reagent described in the literature [Yasaminshirazi et al., 2020], with some modifications. Briefly, 125 μL of the leaven, 125 μL of the FC reagent (diluted at 1:2 with water, v/v), 180 μL of a saturated solution of sodium carbonate, and 1,500 μL of deionized water were mixed and left to react for 1 h at room temperature. Absorbance was measured at 760 nm using a FLUOstar Omega microplate reader (BMG LabTech, Ortenberg, Germany). TPC was expressed in mg of gallic acid equivalent (GAE) per 100 mL of beetroot leaven. The analyses were performed at least in duplicate.
Determination of contents of total betacyanins and total betaxanthins
The content of total betacyanins (expressed as betanin) and total betaxanthins (expressed as vulgaxanthin-I) in leavens was determined spectrophotometrically according to the procedure described by von Elbe [2001]. Leaven samples were diluted with 0.05 M phosphate buffer (pH 6.5) to obtain an absorbance between 0.4 and 0.5 at 538 nm. The light absorption of the samples was recorded at 476, 538, and 600 nm, and the corrected light absorption of betanin and vulgaxanthin-I was calculated according to the equations (5–7) [von Elbe, 2001]:
where: x is absorption of betanin minus colored impurities, y is absorption of vulgaxanthin-I corrected for betanin and impurities, z is absorption of impurities, a is absorption of sample at 538 nm, b is absorption of sample at 476 nm, and c is absorption of sample at 600 nm.Total betacyanin content (TBC) and total betaxanthin content (TBX) were expressed as mg of betanin equivalent (BE) per 100 mL and mg of vulgaxanthin-I equivalent (VG-I) per 100 mL, respectively. All measurements were performed in triplicate.
Statistical analysis
A three-way analysis of variance (ANOVA) by applying a Shapiro-Wilk test was used to assess the effect of the process parameters on the properties of beetroot leaven. Moreover, the correlation coefficient (r) was determined (Pearson test) to analyze the correlations between different parameters of beetroot leaven. All calculations were done at a significance level of α=0.05 using STATISTICA 13.0 software (TIBCO Software Inc., Palo Alto, CA, USA).
RESULTS AND DISCUSSION
Content of total soluble solids and turbidity of beetroot leaven
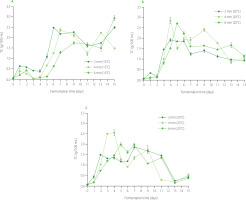
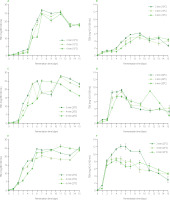
The changes in TSS content in beetroot leaven during fermentation under different conditions are shown in Figure 1. Additionally, regression equations describing these changes are presented in Table S1 in the Supplementary Materials. The TSS content of the brine (fermentation time, 0 day) was 0.18%, and this value increased to 7.25% for the beetroot leaven obtained from the fermentation of 2-mm slices at 15°C for 13 days (Figure 1). Generally, at the beginning of the process, TSS content increased over the fermentation time for all temperature and slice thickness values. The higher the process temperature was, the higher was TSS content after a shorter fermentation time. In terms of slice thickness, the lowest values were noted for 4-mm slices. However, all the highest values of TSS content were noted on or after the 7th day of fermentation. After reaching the maximum, TSS content was observed to decrease. An exception was the leaven from 6-mm beetroot slices fermented at 15°C, for which a linear relationship was found (Table S1). Increasing TSS content during fermentation can be due to the diffusion of fermentable sugars and other solids from beetroot tissue to the surrounding solution (leaven), as well as NaCl and lactic acid from the solution to the material [Rodriguez-Gomez et al., 2012]. Moreover, at the same time, sugars are converted to lactic acid. A sudden decrease in TSS after reaching the maximum value may indicate a significant decrease in the rate of diffusion of the solids from the beet tissue and a significant increase in the rate of decomposition of sugars into lactic acid [Janiszewska-Turak et al., 2022].
Figure 1
Changes in total soluble solid (TSS) content and turbidity (TR) of beetroot leaven during spontaneous fermentation of beetroot slices of different thicknesses at temperatures of 15°C (A and B, respectively), 20°C (C and D, respectively), and 25°C (E and F, respectively).
Turbidity of beetroot leavens ranged from 31 NTU for the product obtained from the fermentation of 6-mm slices at 15°C for 1 day to 1,100 NTU for the leaven from 2-mm slices fermented at 20°C for 13 days (Figure 1). A similar tendency for turbidity changes along with fermentation time can be observed as in the case of TSS, i.e., an increase to the maximum point followed by a decline (Figure 1, Table S1). The linear equations were only found for fermentation of 4-mm and 6-mm slices at 15°C. For all tested slice thicknesses, turbidity reached its highest value the fastest at a temperature of 25°C. Higher temperatures significantly accelerate the rate of lactic acid fermentation and mass exchange during the process. In most cases, the highest turbidity was noted on the same day or two days before the highest content of TSS. It is due to the fact that turbidity is strongly related to the solids content in beetroot leaven and the rate of solid diffusion during the fermentation. Solid substances diffusing from the material into the solution increase its turbidity [Molner et al., 2023].
pH, titratable acidity and total carbohydrate content in beetroot leaven
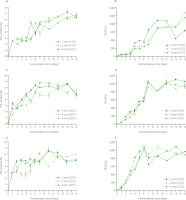
During the lactic acid fermentation of beetroot, the pH decreased from 7.23 for the brine (fermentation time, 0 day) to 3.36 for the leavens from the fermentation of 2-mm slices for 9 days and 6-mm slices for 15 days at 25°C (Figure 2). The lowest pH values were reached by the leaven at the end of the process (13 < fermentation time < 15 day). In general, fermentation allowed for a reduction of pH and the production of a leaven with a pleasant sour taste and a characteristic aroma. However, acidity below pH 3.6 may make the product undesirable in terms of sensory characteristics [Karovicova & Kohajdova, 2003]. From this point of view, more advantageous is achieving pH approx. 4.1, which ensures product stability and, at the same time, its desired sensory properties [Buckenhueskes, 2015]. In our study, depending on the temperature of the process, this pH value was noted between the 4th and 6th day of fermentation (Figure 2). The results are similar to those presented in the literature, where beetroot leaven (natural, without any additions) reached a pH of 4.0 on the 4th day of fermentation [Walkowiak-Tomczak & Zielińska, 2006]. The most intensive changes in pH were observed at the beginning of the process. After two days of fermentation, the pH of the leaven dropped even by 41%, whereas for the next 13 days of fermentation, it changed by 21%. The rapid drop in pH at the beginning of fermentation is important for the quality of the final product, because it minimizes the growth of spoilage bacteria [Viander et al., 2003]. Moreover, in slowly acidified environments, lactic acid bacteria can be inhibited by butyric acid bacteria [Lund et al., 2020].
Figure 2
Changes in pH and titratable acidity (TA) of beetroot leaven during spontaneous fermentation of beetroot slices of different thicknesses at temperatures of 15°C (A and B, respectively), 20°C (C and D, respectively), and 25°C (E and F, respectively). LA, lactic acid equivalent.
Titratable acidity (TA) increased during fermentation from 0.25 g LA/100 mL for the brine to even 11.62 g LA/100 mL for the leavens from the fermentation of 4-mm beetroot slices for 13 days at 25°C. The rate and nature of changes depended both on process temperature and slice thickness (Figure 2; Table S1). The highest TA values were determined at the end of fermentation, i.e., on the 11th day and after. Generally, for all slice thicknesses, it was observed that the higher the process temperature, the higher the TA on specific fermentation days. For example, for 2-mm slices and 7th day of fermentation, the TA values were 4.27, 7.20, and 9.52 g LA/100 mL at temperatures of 15, 20, and 25°C, respectively. It was previously found that the lactic acid fermentation of vegetables and fruits consists of several stages, the first of which is the most important. At this stage, facultative anaerobic microorganisms synthesize carbon dioxide and create conditions for the development of other anaerobic microorganisms, which play a dominant role in subsequent phases [Sun et al., 2021]. Most likely, conducting the process at a higher temperature will result in the acceleration of the metabolism of microorganisms and the faster development of anaerobic conditions. This leads to faster fermentation in further stages, lower pH, a higher concentration of acids (like lactic and formic acids), and therefore higher TA values [Staninska-Pięta et al., 2023].
Total carbohydrate content (TC) of leavens ranged from 0.03 g/100 mL (slice thickness of 6 mm, temperature of 15°C, fermentation time of 3 days) to 2.94 g/100 mL (slice thickness of 6 mm, temperature of 15°C, fermentation time of 15 days) (Figure 3). During fermentation, numerous TC fluctuations were observed, which might have been due to the simultaneous occurrence of two phenomena, i.e., the migration of solid substances (including sugars) from the tissue into the solution and the microbial conversion of sugars into organic acids (mainly lactic acid). Additionally, the brine used to pour the beetroot slices at the beginning of the process (day 0) did not contain sugars; hence, the carbohydrate content in the leaven depended only on the above-mentioned phenomena. Changes in TC are much more difficult to interpret in this case than, e.g., during the lactic acid fermentation of vegetable juices. During this type of fermentation, the material contains large amounts of sugars already before fermentation; thus, their content mainly decreases during the process due to their conversion by lactic acid bacteria to lactic acid [Manea & Buruleanu, 2010]. However, a certain influence of temperature on the kinetics of TC changes in leavens during beetroot fermentation was observed (Figure 3; Table S1). In the case of the samples subjected to fermentation at 15°C, the highest TC were achieved on 7 < fermentation time < 15 day, while at 20 and 25°C it was on 4 < fermentation time < 7 day. During fermentation, sugars are partially extracted into the brine, and temperature is one of the main factors affecting that mechanism. Generally, higher temperatures induce higher water extraction efficiency for sugars [Lopez et al., 2009]. In this case, it can be noted that with increasing temperatures of fermentation, the rate of extracted sugars was higher, and the highest values obtained for TC were observed in increasingly earlier stages of fermentation (Figure 3).
Color of beetroot leaven
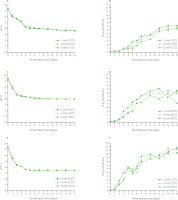
Figure 4 shows changes in leaven color parameters during the fermentation process. In all tested samples, the greatest color changes (ΔE) occurred in the first few days of fermentation. This was most likely closely related to the greater intensity of pigment release into the brine at the beginning of fermentation. Moreover, the greatest drop in pH was observed during the first 2 days, which may also have a significant impact on the color of the leavens. As the fermentation temperature increased, the highest ΔE values appeared earlier and were recorded on the 4th, 2nd, and 1st day of fermentation for temperatures of 15, 20, and 25°C, respectively. Moreover, with an increase in the fermentation temperature, the maximum L*, a*, b*, and ΔE values of leavens decreased (Figure 4). For example, leaven redness (a*) ranged from 34.92 to 60.67, from 32.03 to 57.60, and from 28.57 to 47.93 for fermentation temperatures of 15, 20, and 25°C, respectively. It indicates faster decomposition of red colorants at higher fermentation temperature, which is consistent with the literature data [Staninska-Pięta et al., 2023].
Figure 4
Changes in color coordinates and total color difference (ΔE) of beetroot leaven during spontaneous fermentation of beetroot slices of different thicknesses at temperatures of 15°C (A and B, respectively), 20°C (C and D, respectively), and 25°C (E and F, respectively). L*, lightness; a*, (+)redness/(−)greenness; b*, (+)yellowness/(−)blueness.

Content of total phenolics, total betacyanins and total betaxanthins in beetroot leaven
During fermentation of beetroot, total phenolic content in the leaven increased from 1.12 mg GAE/100 mL (slice thickness of 6 mm, temperature of 25°C, fermentation time of 1 day) to even 103.93 mg GAE/100 mL (slice thickness of 4 mm, temperature of 25°C, fermentation time of 4 day) (Figure 5). Generally, in all tested samples, the lowest TPC was recorded on the 1st day of fermentation, and the greatest increase in its value was observed at the very beginning of the process, i.e., between days 1 and 2 (even by 97%). Then, there was a further increase in TPC (with numerous fluctuations) at a rate dependent on the process temperature. Phenolic compounds of beetroots, which include flavonoids and phenolic acids [Płatosz et al., 2020], are important secondary metabolites of plant and have highly varied structures and properties. Increased TPC in beetroot leaven during fermentation may be related to microorganisms’ ability to break down the food matrix, releasing associated phytochemicals. These microorganisms produce enzymes like cellulase, β-glucanase and lichenase capable to cleave the glycosidic bounds and release phenolic acids from plant cell walls [Matthews et al., 2006]. Breaking these bonds contributes to the release of conjugated acids, which is expressed in a higher TPC [Płatosz et al., 2020].
Figure 5
Changes in total phenolic content (TPC) of beetroot leaven during spontaneous fermentation of beetroot slices of different thicknesses at temperatures of 15°C (A), 20°C (B), and 25°C (C). GAE, gallic acid equivalent.
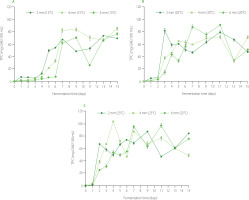
Betalains are classified based on structural characteristics into betacyanins (betanin and isobetanin) with red-violet coloration, which account for 75–80% and betaxanthins (vulgaxanthin-I, II) with yellow-orange coloration, which constitute 20–25% of total betalains present in beetroot [Guine et al., 2018]. In the present study, changes in total betacyanin content and total betaxanthin content of the leaven during fermentation are presented in Figure 6. TBC and TBX ranged from 0.27 mg BE/100 mL (slice thickness of 6 mm, fermentation at 15°C for 1 day) to 27.96 mg BE/100 mL (slice thickness of 2 mm, fermentation at 20°C for 11 days) and from 0.39 mg VG-I/100 mL (slice thickness of 6 mm, fermentation at 20°C for 1 day) to 12.15 mg VG-I/100 mL (slice thickness of 2 mm, fermentation at 25°C during 5 days), respectively. These results agree with literature data for red (from 0.9 to 32.3 mg/100 g) and yellow (from 1.5 to 15.1 mg/100 g) pigment contents in natural leaven [Walkowiak-Tomczak & Zielińska, 2006]. Changes in TBC and TBX during fermentation showed a similar trend, i.e., the contents of the analyzed betalains increased to their maximum value, and then their fluctuations with a tendency to decrease were observed (Figure 6; Table S1). For example, the TBC of the leaven from the fermentation of 6-mm slices at 20°C increased during the first seven days from 0.31 to 25.06 mg BE/100 mL, and then decreased in the following days of processing to 16.20 mg BE/100 mL on the 15th day of fermentation. Decreasing or increasing contents of both betacyanins and betaxanthins in the leaven during the fermentation of beetroot resulted from the intensity of two processes occurring, i.e., the release of pigments from the matrix and their degradation. At the beginning of the process, when the leaching of pigments from the food matrix was dominant, an increase was observed in betacyanin content. Next, when these compounds reached their maximum concentrations, the most likely mechanism of their degradation started to be a leading one. The sudden increases in TBC and TBX in the periods with a decreasing tendency may result from a temporary higher leaching efficiency of the compounds due to more intensive maceration of the inner layers of beetroot slices [Sawicki & Wiczkowski, 2018].
Figure 6
Changes in contents of total betacyanins (TBC) and total betaxanthins (TBX) of beetroot leaven during spontaneous fermentation of beetroot slices of different thicknesses at temperatures of 15°C (A and B, respectively), 20°C (C and D, respectively), and 25°C (E and F, respectively). BE, betanin equivalent; VG-I, vulgaxanthin-I equivalent.
Effect of process parameters on beetroot leaven properties
Effect of process parameters and interactions between them on beetroot leaves properties was analyzed by three-way ANOVA. Respective results are presented in Table 1. All independent variables had a significant effect on all beetroot leaven properties (dependent variables). Considering the significance of the influence of parameters alone (without their interactions), the order is as follows: fermentation time (FT) > temperature of fermentation (T) > slice thickness (ST), and the % of explained variance in these cases ranged from 33.87% (total carbohydrate content) to 83.83% (total betacyanin content), from 1.37% (total carbohydrate content) to 27.79% (total betaxanthin content), and from 0.01% (pH) to 4.03% (L*), respectively. Overall, the fermentation time itself turned out to be the most important process parameter, accounting for 75% of all studied properties. In the other 25% (for a*, total carbohydrate content, and ΔE), it was the second most significant variable, just after the combination of fermentation time and temperature (FT×T).
Table 1
Results (% of the explained variance) of three-way analysis of variance showing the effects of slice thickness (ST), fermentation time (FT) and temperature (T) as well as their interactions on physicochemical parameters of beetroot leavens.
[i] *Significant at p<0.05; ns, not significant at p≥0.05; TSS, total soluble solid content; TR, turbidity; TA, titratable acidity; TC, total carbohydrate content; TPC, total phenolic content; TBC, total betacyanin content; TBX, total betaxanthin content; L*, lightness; a*, (+)redness/(−)greenness; b*, (+)yellowness/(−)blueness; ΔE, total color difference; LA, lactic acid equivalent; GAE, gallic acid equivalent; BE, betanin equivalent; VG-I, vulgaxanthin-I equivalent.
In the case of the impact of the interactions of process parameters, their order in terms of significance was as follows: FT×T > ST×FT×T > ST×FT > ST×T, where the % of explained variance ranged from 5.12% (titratable acidity) to 44.49% (a*), from 0.16% (pH) to 13.42% (total soluble solid content), from 0.07% (turbidity) to 6.21 % (total carbohydrate content), and from 0.04% (pH) to 2.34% (total carbohydrate content), respectively (Table 1).
Correlations between beetroot leaven properties
Lactic acid fermentation is a complex process, and the tested properties of the leaven were influenced not only by the parameters of the process itself, but also by their interactions. For a better understanding of the interrelationships between all the analyzed properties of beet leaven, Pearson correlation coefficients were determined and presented in Table 2. All correlations were significant (p<0.05), and the correlation coefficients were in the range of 0.31≤|r|≤0.97.
Table 2
Coefficients of Pearson correlations of physicochemical parameters of beetroot leaven obtained during fermentation at different conditions.
[i] *Significant correlation at p<0.05. TSS, total soluble solid content; TR, turbidity; TA, titratable acidity; TC, total carbohydrate content; TPC, total phenolic content; TBC, total betacyanin content; TBX, total betaxanthin content; L*, lightness; a*, (+)redness/(−)greenness; b*, (+)yellowness/(−)blueness; ΔE, total color difference.
The positive correlations between the parameters of beet leaven with the highest coefficients (r≥0.80) were recorded for a* vs. ΔE (r=0.97), L* vs. ΔE (r=0.94), L* vs. a* (r=0.93), b* vs. ΔE (r=0.89), TR vs. TA (r=0.87), L* vs. b* (r=0.84), TR vs. TBC (r=0.83), TA vs. TBC (r=0.81), TPC vs. TBC (r=0.81), a* vs. b* (r=0.81), and TSS vs. TBC (r=0.80). Apart from the expected high correlations between color parameters (0.89≤r≤0.97) of the beetroot leaven, the relationship between TR vs. TA also showed a high positive correlation (r=0.87). This can be due to the fact that lactic acid produced by lactic acid bacteria leads to acidification of the environment and increases the TA of beetroot leaven. At the same time, the growing lactic acid bacteria population resulted in an increase in leaven turbidity [Reina et al., 2005]. Most probably, a strong positive correlation between TR vs. TBC and TSS vs. TBC (Table 2) was related to the leaching of betalains into the surrounding solution during the fermentation process. These extracted compounds enrich the total extract present in the leaven while, at the same time, increasing its turbidity [de Jesus Junqueira et al., 2018]. In addition, betanin is more stable in an acidic environment. During fermentation, when the TA of beetroot leaven increased, betanin became more stable; hence, TBC content increased. A similar effect of a positive correlation between titratable acidity and content of betanins in beetroot leaven was previously noted by Sadowska-Bartosz & Bartosz [2021].
In the case of the strongest negative correlations (−r≥0.80), they were observed between pH vs. TR (r=−0.80), pH vs. ΤΑ (r=−0.80), and pH vs. TBC (r=−0.80) (Table 2). Due to the fact that both pH and TA indicate the acidification of the environment (with opposite phrases), there was such a strong negative correlation between these two properties of the leaven. Additionally, for the same reason, high negative correlations between pH vs. TR and TBC should be interpreted in the same way as high positive correlations between TA vs. TR and TBC.
As can be seen in Table 2, two individual parameters of the beetroot leaven strongly correlating with all other parameters were TPC (0.64≤|r|≤0.81) and L* (0.61≤|r|≤0.94). On the other hand, the beetroot leaven parameters characterized by the largest number of weak correlations (0.3<|r|<0.5) were TC (8 out of 11) and TBX (7 out of 11).
CONCLUSIONS
In this work, beetroot slices with a thickness of 2, 4, and 6 mm were subjected to spontaneous lactic acid fermentation at 15, 20, and 25°C for a maximum of 15 days to determine the most desirable conditions in terms of physicochemical properties and color of the resulting leaven. Although all variables had a significant impact on the properties of beet leaven, the fermentation time and temperature were of key importance. Study results showed that a preferable pH below 4.1 was achieved in leavens between 4 and 6 days of fermentation. However, titratable acidity reached the highest values only in the final phase of fermentation. In contrast, total carbohydrate content was generally the highest between 4 and 7 days of fermentation. To obtain a leaven with higher total soluble solids and turbidity, beetroots need to be fermented for at least 7 days. In order to increase the content of bioactive compounds in the leaven, the most advantageous process parameters were thinner beetroot slices (4 mm for total phenolic content and 2 mm for total betacyanin content) and higher fermentation temperature (20°C−25°C). The highest contents of total phenolics, total betaxanthins and total betacyanins were determined in the leaven on the 4th, 5th and 11th day of fermentation, respectively. On the other hand, the highest values of color parameters (L*, a*, b*, and ΔE) were obtained when thicker beetroot slices (4-mm and 6-mm) were fermented at lower temperature (15°C) for 4 days. The presented research allows for the selection of parameters of the beetroot fermentation process focusing on the specific properties of the leaven (their maximum or minimum values). However, in terms of all tested properties, the optimal process parameters were determined at: slice thickness of 6 mm, fermentation temperature of 20°C and fermentation time of 7 days.