INTRODUCTION
The mango (Mangifera indica L.) belongs to the Anacardiaceae family, it is cultivated in the tropical areas, with temperature being an important factor in its growth and development. Currently, mango is the most consumed fruit worldwide, called the “King fruit” [Jahurul et al., 2015]; likewise, it is the third most traded tropical fruit in terms of volume [FAO, 2023]. In Peru, the largest production of mangoes for export is concentrated in the geographical area of Piura. Among them is the Criollo variety, intended for the production of pulp and concentrated juices, and the Haden, Kent, Tommy Atkins and Edward varieties, intended for consumption as fresh fruit. The main export countries of Peruvian mango, for the period 2023/24, were the Netherlands (40% of national production) followed by the United States (30%), Spain (8%), the United Kingdom (6%) and others (16%) [Agencia Agraria de Noticias, 2024]. However, mango leaves are considered waste material generated mainly by pruning, but they are an important resource for their health benefits due to the abundance of phytochemicals such as mangiferin, phenolic acids, benzophenones, flavonoids, ascorbic acid, carotenoids, tocopherols and chlorophylls [Kumar et al., 2021]. These phytochemicals foster high potential in terms of biological and pharmacological activities such as antioxidant, antidiabetic, anti-inflammatory, antimicrobial, antiviral, immunomodulatory, antiobesity, antiallergic, antifungal, antiparasitic, antipyretic, hepatoprotective, anti-microbial, antidiarrheal, antitumor, and others [Batool et al., 2018]. Among them, phenolic compounds are very important. Different epidemiological and experimental studies have demonstrated the protective effect of polyphenols against chronic diseases such as cancer, diabetes as well as cardiovascular and neurode-generative diseases [Rasouli et al., 2017]. Polyphenols modulate numerous physiological processes, such enzyme activity, cell proliferation, signal transduction pathways and cellular redox potential to fight chronic pathologies [Luca et al., 2020].
Bread is a widely consumed food and is part of an everyday diet in different populations around the world. Making bread basically includes wheat flour, water and a small amount of salt. The impact of industrialization on bread making has allowed the addition of other materials such as food additives and yeast to improve bread quality characteristics. Currently, there is a great interest in the enrichment of formulations with various plant flours in order to improve bread nutritional and bioactive properties. The partial replacement of wheat flour with plant-based ingredients has favorably contributed to the increased contents of protein, polyunsaturated fatty acids, polyphenols, plant pigments, and enhanced bioactivity, e.g., antioxidant capacity [Amoah et al., 2022; Kowalski et al., 2020].
Kumar et al. [2021] considered the mango leaf powder as a potential resource for the development of functional foods due to the bioactive potential. However, there are few research works that have used this by-product as a partial substitute in food products [Yelwande et al., 2022]. An approach to the utilization of mango leaves is based on the development of functionalized edible films made of chitosan/starch with a mango leaf extract, and the development of sustainable preservatives to avoid the perishability of climacteric fruits [Cejudo et al., 2023; Majeed et al., 2024].
Therefore, the main objective of the research carried out was to develop a sliced bread (SB) enriched with mango leaf powder (MLP) with enhanced antioxidant capacity and increased phenolic compound content, as well as acceptable sensory and texture properties.
MATERIALS AND METHODS
Plant materials
Leaves of three varieties (Criollo, Kent, and Edward) of Mangifera indica L. were collected between February and March 2022 at fruit farms of the Association of Mango Producers of the province of Chulucanas (Piura, Peru). GPS coordinates of the leaf collection site were 5°5′43.692″S and 80°10′10.56″W; at an altitude of 92 m above sea level. Approximately 7 kg of young leaves were taken from the bottom of 6 trees for each mango variety studied.
Mango leaf powder preparation
The preliminary post-harvest operations consisted of leaf selection, washing, disinfection and drying at 60°C for 4 h using a commercial dryer with 15 stainless steel trays. Disinfection was carried out by immersing the leaves for 10 min in a diluted solution (5 mL/L of water) of Bio Organic® (Econo Group E.I.R.L, Lima, Perú). The leaves were then milled using a blade mill with a sieve of 0.5 mm in diameter. The MLP was packed in polythene bags and stored until it was used to produce SB and for chemical analysis.
Sliced bread preparation
Sliced breads (SBs) were prepared with wheat flour (control) and with partial replacement of wheat flour (2%, 6%, and 10%) with MLP. In addition to wheat flour (42%, 46%, 50% and 52%) and MLP (10%, 6%, 2% and 0%, respectively), the breads were made of milk (35%), sugar (2%), yeast (1%), butter (9%), and salt (1%). MLP was mixed with the dry ingredients and then with the rest of the ingredients. The kneading time was 8 min. The dough was then left to rest in containers until doubling in size, i.e., for about 1 h. Subsequently, the dough was placed in the stainless-steel molds of 25×11×6 cm, fermented for 2 h at room temperature (20°C), and then baked at 230°C for 35 min. Finally, SBs were allowed to cool to room temperature and packaged in polypropylene bags. Formulations of SB control (0% MLP); SB+Criollo (2%, 6% and 10% MLP), SB+Kent (2%, 6% and 10% MLP) and SB+Edward (2%, 6% and 10% MLP) were prepared in triplicate, and SBs were made twice – the first time for chemical and texture analyses and the second time for sensory evaluation and color measurements.
Preparation of methanolic extracts of mango leaf powder and sliced bread
For the preparation of methanolic extracts of MLP, the procedure described by Romero-Orejón et al. [2023] was followed with some modifications. Approximately 0.5 g of MLP was placed in 15-mL centrifuge tubes, and then 5 mL of a methanol and water mixture (70:30, v/v) was added. In the case of SB, 10 g was weighed into a 125-mL Erlenmeyer flask, and 50 mL of a methanol and water mixture (70:30, v/v) was added. The extraction was developed in two stages, the first by vortexing at the maximum speed for 30 min, and the second by ultrasound bath (CPXH 2.8 L, Bransonic, Danbury, CT, USA) at 40 kHz and 30°C for 30 min. Subsequently, the extracts were centrifuged at 4,000×g for 15 min, and the supernatant was recovered and stored at 5°C until further analyzed.
Determination of total phenolic content
The total phenolic content (TPC) was determined following the conventional method with a Folin-Ciocalteu reagent [Singleton & Rossi, 1965] with some modifications made by Martínez & Ramos-Escudero [2024]. A 50-μL aliquot of MLP or SB extract was reacted with 0.38 mL of the Folin-Ciocalteu phenol reagent (0.2 M). The reaction was developed for 5 min, and subsequently 0.38 mL of 7.5% sodium carbonate was added. The blue color complex was allowed to develop for 2 h, after this time the absorbance readings were collected at 765 nm using an OrionTM AquaMate 8100 UV-Visible spectro-photometer (Thermo Scientific, Waltham, MA, USA). The results were expressed in mg of gallic acid equivalent per g of MLP or SB (mg GAE/g).
Determination of total flavonoid content
The total flavonoid content (TFC) was determined by the method described by Miliauskas et al. [2004] with some modifications. A 10-μL aliquot of MLP or SB extract was mixed with 0.1 mL of aluminum chloride hexahydrate (80 mM) and then diluted with 1 mL of distilled water. Absorbance readings were recorded at 415 nm after 15 min at room temperature. The total flavonoid content was expressed as mg of rutin equivalent per g of MLP or SB (mg RE/g).
Determination of plant pigment content
The plant pigments of the samples were determined following the methodology described by Lichtenthaler & Buschmann [2001]. Approximately 0.1 g of MLP or SB was weighed and then hydrated with 0.2 mL of water for 3 min. Then, the extraction of chlorophylls and carotenoids was developed by the addition of 5 mL of an acetone and water mixture (80:20, v/v). The extraction continued with stirring by vortex and ended with centrifugation at 4,000×g for 15 min. The supernatant was separated and the spectrum in the wavelength range of 400–700 nm was recorded using an Orion AquaMate 8100 UV-visible spectro-photometer (Thermo Scientific). The wavelengths selected for quantification of chlorophylls were 646 and 664 nm, whereas carotenoids were quantified at the wavelength of 470 nm. The total content of individual pigment types was calculated using equations described by Lichtenthaler & Buschmann [2001]. The total content of chlorophylls and carotenoids was expressed in mg per g of MLP or SB.
Determination of ferric ion-reducing antioxidant power
Ferric ion-reducing antioxidant power (FRAP) of MLP or SB was determined following the method described by Benzie & Strain [1996] with some modifications. A 0.75-mL aliquot of a solution containing a mixture of 2.5 mL of 2,4,6-tris(2-pyridyl)-s-triazine (10 mM in 40 mM HCl), 2.5 mL ferric chloride (20 mM), and 25 mL of sodium acetate (300 mM adjusted to pH 3.6) was added to a 2-mL Eppendorf tube, and then 0.12 mL of a previously diluted MLP or SB extract was added. Absorbance readings were collected at 593 nm. Results were expressed in μmol FeSO4/g of MLP or SB.
Determination of 2,2-diphenyl-1-picrylhydrazyl radical scavenging activity
2,2-Diphenyl-1-picrylhydrazyl (DPPH) assay was carried out following the procedure described by Brand-Williams et al. [1995]. An amount of 10 μL of an MLP or SB extract diluted with 40 μL of a methanol and water mixture (70:30, v/v) was reacted with 950 μL of the DPPH radical solution (100 μM). Absorbance readings were collected at 515 nm after 10 min of reaction. DPPH radical scavenging activity was expressed as μmol Trolox equivalent per g of MLP or SB (μmol TE/g).
Color measurements
Color measurements of the SB crumb were carried out by image analysis following the procedure described by Zegarra et al. [2019]. The samples were placed in a portable photo studio (Puluz Technology Limited, Shenzhen, China) containing 64 LED lights with an output power of 30 W and a color temperature of 5,500 K. The photographic images were acquired using a digital camera (Canon, Power Shot SX60 HS, full HD 65X optical zoom, Tokyo, Japan). The photographs were saved on a memory card, and then the RGB color squares were obtained by means of the ImageJ-1.51k software (National Institutes of Health by Wayne Rasband, USA). The L* (darkness/whiteness), a* (greenness/redness) and b* (blueness/yellowness) values were obtained using the color matching tool (Nix Sensor) (https://www.nixsensor.com/free-color-converter/), while the total color difference (ΔE) was estimated from Equation (1):
where: ΔL*, Δa*, and Δb* are the differences in the L*, a* and b* coordinates, respectively, between SB control and SB enriched with MLP.Evaluation of sensory properties
A sensory analysis of bread samples was conducted 3 to 5 h after baking with a panel of 15 trained judges from the academic program of food industry engineering and agribusiness, consisting of 6 men and 9 women aged 25 to 45. They evaluated attributes like color, taste, and odor for nine coded treatments, scoring preferences from 9 (best) to 1 (worst). The Friedman classification test was used for discrimination between the samples. Judges signed informed consent, and the study adhered to the ethical principles of the Declaration of Helsinki.
Texture profile analysis
Texture profile analysis (TPA) assessed attributes such as hardness, cohesiveness, springiness, adhesiveness, chewiness, and gumminess, using an analyzer from Ametek (Middleborough, MA, USA). A plexiglass probe with a 25 mm diameter and 35 mm height was employed. The settings included a pretest speed of 1.0 mm/s, a test speed of 2.0 mm/s, a firing force of 0.05 N, and 60% strain, conducted over two compression cycles with a 5-s pause. Five slices from each SB formulation, each 15 mm thick, were evaluated, with data collected at three points per slice. The equipment utilized TexturePro CT Advanced Edition software (21 CFR, Ametek), for data analysis.
Statistical analysis
Results were presented as the mean and standard deviation (SD). An analysis of variance (ANOVA) followed by Fisher’s least significant difference (LSD) test was used to compare means between varieties of MLP and in SBs with different formulations. Differences were considered significant at p<0.05. For sensory evaluation, multiple non-parametric comparisons were used using Friedman classification test and Kendall’s coefficient of agreement (KCC) to measure agreement among judges; and the Newman-Keuls statistical test (N-K) was used to evaluate differences between formulations [Meilgaard et al., 1999; Scheff, 2016]. The statistical package STATISTICA, version 8.0 (StatSoft, Inc., Tulsa, OK, USA), was used to analyze all data.
RESULTS AND DISCUSSION
Bioactive compound contents and antioxidant capacity of mango leaf powder
The content of bioactive compounds in MLP is shown in Table 1. The TPC of the leaf powder from three mango varieties varied between 63.93 to 76.52 mg GAE/g, and no significant difference (p≥0.05) was found between the varieties Edward and Kent; the variety Criollo was the one that presented lower value. In this regard, Fernández-Ponce et al. [2016] found a TPC of ~12 mg GAE/g in dehydrated mango leaves of the Kent variety, however, Sultana et al. [2012] found values of 86.62 and 93.18 mg GAE/g of mango leaves of Langra and Chonsa varieties, respectively. Likewise, Kingne et al. [2018] indicated that the state of maturity of mango leaves influenced the TPC, demonstrating that it varied between 40 to 90 mg GAE/g with the youngest leaves featuring a higher TPC content compared to the mature leaves. The results of this research demonstrate that leaves of Criollo, Kent and Edward varieties had a high TPC and when compared to other species were slightly similar to cat’s claw leaves (~65 mg GAE/g), green tea (~89 mg GAE/g) [Chacaliaza-Rodríguez et al., 2016], leaves of different grape varieties (~27 to ~76 mg GAE/g) [Pantelić et al., 2017], and superior to quinoa leaves (~10 mg GAE/g), and Erythroxylum coca var. coca leaf (~33.02 mg GAE/g) [Chacaliaza-Rodríguez et al., 2016].
Table 1
Contents of total phenolics, total flavonoids and plant pigments of leaf powders from three mango varieties.
The TFC, in the mango leaves evaluated, was in the range of 37.36 to 65.14 mg RE/g (Table 1). The MLP from Criollo variety presented higher TFC compared to the Edward and Kent varieties. These results differed from the findings reported in other studies. For example, Ghosh et al. [2022] reported TFC of mature leaves from various mango varieties between ~0.5 and ~3.5 mg quercetin equivalent (QE)/g. In addition to using another reference than in our study (quercetin vs. rutin), they mentioned that the recovery capacity of the flavonoid, which is associated with the type of solvent used for extraction, influenced the determined flavonoid content of mango leaves. The extraction with methanol was more effective than the extraction with acetone and petroleum benzine, indicating that the flavonoids of mango leaves have polar characteristics. Kingne et al. [2018] found that the total flavonoid content of young and mature mango leaves varied from 6 to 14 mg catechin equivalent (CE)/g, thus demonstrating that the young leaves presented higher values than the mature leaves. On the contrary, Sultana et al. [2012] reported TFC between 76.54 and 83.67 mg CE/g of leaves for Langra and Chonsa varieties, respectively. The TFC of mango leaves of Kent, Edward and Criollo varieties, determined in our study, was superior compared to other edible leaf species, like for example, leaves of different mint species (3.65 to 16.83 mg QE/g dry weight, dw) [Ćavar Zeljković et al., 2021], basil (~12 mg QE/g dw), chard (~11 mg QE/g dw), parsley (~15 mg QE/g dw) and red kale (~7 mg QE/g dw) [Chandra et al., 2014]. The main flavonoids identified in mango leaves were catechin, rutin, kaempferol 3-O-rutinoside, isoquercitrin, hyperin, quercetin 3-O-glucoside and quercetin [Kumar et al., 2021; Sferrazzo et al., 2022].
The plant pigments, including especially carotenoids and chlorophylls, were mainly responsible for the color in MLP. The total carotenoid content ranged from 0.54 to 0.69 mg/g, while the total chlorophyll content ranged from 2.04 to 2.48 mg/g (Table 1). The total carotenoid content showed significant differences (p<0.05) between the varieties of Kent, Edward, and Criollo, while in respect to the total chlorophyll content, the varieties of Kent and Edward were statistically similar (p≥0.05). Sousa [2022] have mentioned that the leaves of edible plants contain significant amounts of plant pigments, and that the most common are chlorophyll, carotenoids, and antho-cyanins. In addition, plant pigments have demonstrated multiple biological effects including antitumor, anti-atherogenic, and anti-inflammatory activity [Magalhães et al., 2024]. Mango leaves of Kent, Edward, and Criollo varieties were slightly lower in plant pigment content than leaves of quinoa varieties, showing from ~0.64 to ~0.91 mg/g of carotenoids and from 2.02 to 3.91 mg/g of chlorophylls [Chacaliaza-Rodríguez et al., 2016]. Likewise, the content of total plant pigments was reported at ~0.42 to ~2.41 mg/g sample in the commercial samples of coca leaves, lemon verbena, cats’ claw, and green tea [Chacaliaza-Rodríguez et al., 2016].
The antioxidant capacity of MLP from different mango varieties determined by the DPPH and FRAP assays is shown in Table 2. For the DPPH assay, values varied from 19.09 to 47.45 μmol TE/g, while the FRAP fluctuated between 80.25 to 107.71 μmol FeSO4/g. Regarding antioxidant capacity, the mango leaf varieties presented the following order: Kent > Edward > Criollo. Results of the DPPH assay were similar to those determined for other edible leaves such as spinach, mustard, and moringa (21.8 to 60.6 μmol TE/g), while for chili leaves and lettuce red coral they ranged from 197.3 to 380.6 μmol TE/g [Manaois et al., 2020]. The antioxidant capacity of mango leaves confirmed the results of a previous study by Sferrazzo et al. [2022], who stated that mango leaves are an excellent source of phenolic compounds that contribute to their antioxidant properties and can scavenge free radicals.
Total phenolic content, total flavonoid content, and antioxidant capacity of sliced bread enriched with mango leaf powder
The TPC, TFC, and antioxidant capacity determined through DPPH and FRAP assays of sliced breads produced from different recipes are summarized in Table 3. The TPC and TFC of the control SB without MLP were 0.58 mg GAE/g and 0.08 mg RE/g, respectively, and the antioxidant capacity determined by DPPH and FRAP assays was 0.02 μmol TE/g and 0.25 μmol FeSO4/g, respectively. These values were significantly (p<0.05) higher for the breads enriched with MLP and increased with increasing MLP content in the bread recipe. Moreover, it was observed that among SBs produced with the highest percentage of MLP in the recipe (10%), the MLP from Kent variety allowed obtaining the most advantageous product in terms of TPC, TFC and anti-oxidant capacity.
Table 3
Total phenolic content, total flavonoid content and antioxidant capacity of wheat flour sliced bread (SB control) and sliced breads (SB) enriched with leaf powders (replacement of 2%, 6%, and 10% of wheat flour) from three mango varieties (Criollo, Kent and Edward).
[i] Values (mean ± standard deviation. n=3) with different letters in the same column indicate a significant difference at p<0.05, according to Fisher’s LSD test. GAE, gallic acid equivalent; RE, rutin equivalent; DPPH assay, assay with 2,2-diphenyl-1-picrylhydrazyl radical; FRAP, ferric ion-reducing antioxidant power; TE, Trolox equivalent.
Leaves from plant sources have been used for bread fortification to achieve healthier foods rich in plant metabolites such as carotenoids, chlorophylls, and flavonoids [Amoah et al., 2022]. For example, quercetin and kaempferol derivatives have been detected in slices of bread enriched with pea leaf, while slices of bread enriched with lupine leaves have been found rich in chrysoeriol and genistein that could elicit health benefits, especially in the treatment of women’s diseases [Klopsch et al., 2018; Yu et al., 2021]. Another report mentioned that the enrichment of bakery products with stinging nettle leaves (Urtical dioica) contributed to an increase in lutein, β-carotene, and total phenolics content and also in antioxidant activity [Maietti et al., 2021]. These findings indicate that the fortification of bakery products with plant sources improves their nutritional and functional properties.
There is a little evidence in the scientific literature on the commercial application of the use of MLP even though the MLP extract has been shown to provide excellent antioxidant capacity and antimicrobial activity towards Pseudomonas fluorescens, Staphylococcus haemolyticus, and Staphylococcus aureus [Sferrazzo et al., 2022]. On the other hand, an in vitro cytotoxicity study with human hepatic stellate cell (LX-2) and macrophages (U937) has not shown the evidence of cell viability at concentrations between 35 to 150 μg/mL [Sferrazo et al., 2022]. One approach for the use of the MLP extract in the food industry is the development of active packaging based on films composed of polymers and polyphenols of MLP for the extension of the shelf life of food products [Cejudo-Bastante et al., 2021, 2022].
Chromatic parameters of sliced bread enriched with mango leaf powder
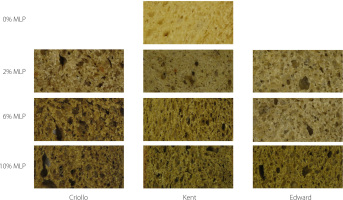
Color is one of the most important parameters in determining the acceptability of food by consumers. The chromatic parameters of SB enriched with MLP are shown in Table 4, and photos showing SB appearance are presented in Figure 1. Lightness was higher for SB control (65.26) in comparison with SBs enriched with MLP (40.71 to 59.10). However, the SB+Edward 2% did not differ significantly (p≥0.05) from SB control. L* values of SB enriched with 2% MLP ranged from 51.79 to 59.10, while those of SB with 6% MLP ranged from 48.01 to 52.34. The color of the SB darkened with increasing percentage of MLP in the bread recipe. This relationship was also observed in biscuits enriched with 1% and 2% of Spirulina biomass with L* values of 42.86 and 36.62, respectively [Şahin, 2020]. The reduction in lightness affected the other color parameters. The chromatic coordinate a* ranged from 0.93 (SB control) to 4.55 (SB+Criollo 10%) except for the formulation SB+Kent 10% that presented a negative value (−1.85) (Table 4). The a* value was significantly (p<0.05) higher compared to the control only for two samples (SB+Criollo 6% and SB+Criollo 10%). The value of a* did not differ significantly (p≥0.05) between most of samples. In this section, it is important to emphasize that the MLP color presented negative a* values ranging from −6.98 to −9.75 (data no shown), indicating a greenish color. It is possible that the positive a* values of most SBs were due to the depletion of plant pigments; hence, the formation of pheophytin [Klopsch et al., 2018]. For the chromatic parameter b*, it was observed to be lower in SBs with MLP (31.41 to 41.97) compared to the control SB (43.92). These results are consistent with those reported by Cacak-Pietrzak et al. [2023] who found a decrease in b* values when 1% to 6% of freeze-dried pomace from black chokeberry (Aronia melanocarpa L.) was added to bread. The same decrease was observed in food matrices rich in chlorophyll as in biscuits enriched with Spirulina biomass, while biscuits enriched with Dunaliella biomass rich in β-carotene showed an increase in b* values [Şahin, 2020]. Finally, the total color difference showed an increase when increasing the proportion of 2%, 6%, and 10% of MLP in SB recipe with values that fluctuated between 13.90 and 26.32.
Table 4
Chromatic parameters of wheat flour sliced breads (SB control) and sliced bread (SB) enriched with leaf powders (replacement of 2%, 6%, and 10% of wheat flour) from three mango varieties (Criollo, Kent and Edward).
Sensory properties of sliced bread enriched with mango leaf powder
The sensory evaluation determined the degree of acceptance of breads enriched with MLP according to the attributes of color, flavor, and odor, i.e., features that determine consumer choice when purchasing bread. Table 5 shows the values assigned to SBs in their sensory evaluation. The results shown for the attributes of color, taste, and odor indicate significant differences between the products, finding greater acceptability of SBs enriched with 2% of MLP in the recipe. Regarding color, the judges preferred lighter and less greenish colors that were intensified by carotenoids and chlorophylls, with an increase in the percentage of wheat flour substitution by MLP in the SB recipe. However, in the evaluation of taste and odor, there were no significant (p≥0.05) differences between the SBs with 2% and 6% of MLP from the Criollo and Kent varieties. This may be because dark breads tend to be associated with a burnt bread taste and green hue of bread is associated with a bitter taste.
Table 5
Sensory scores of sliced breads (SB) enriched with leaf powders (replacement of 2%, 6%, and 10% of wheat flour) from three mango varieties (Criollo, Kent and Edward).
| Bread | Sum of color attribute ranks | Color | Sum of taste attribute ranks | Taste | Sum of odor attribute ranks | Odor |
|---|---|---|---|---|---|---|
| SB+Criollo 2% | 108 | 7.20±2.04a | 109 | 7.26±2.34a | 86 | 5.80±2.56ab |
| SB+Kent 2% | 113 | 7.53±1.40a | 97 | 6.46±1.99ab | 92 | 6.13±2.58ab |
| SB+Edward 2% | 113 | 7.53±1.88a | 103 | 6.86±2.09a | 113 | 7.53±1.84a |
| SB+Criollo 6% | 78 | 5.20±2.33b | 91 | 6.06±1.98abc | 77 | 5.13±2.69bc |
| SB+Kent 6% | 71 | 4.73±1.38b | 73 | 4.86±2.16bcd | 76 | 5.20±2.62bc |
| SB+Edward 6% | 72 | 4.80±1.61b | 67 | 4.46±1.95cde | 59 | 4.00±2.32bc |
| SB+Criollo 10% | 34 | 2.26±1.16c | 54 | 3.60±1.99de | 59 | 3.93±2.21bc |
| SB+Kent 10% | 56 | 3.73±1.75b | 42 | 2.80±1.89e | 66 | 4.40±2.16bc |
| SB+Edward 10% | 30 | 2.00±1.30c | 39 | 2.60±1.95e | 45 | 3.00±1.55c |
| Friedman X2 | 71.804 | 49.546 | 29.958 | |||
| p-value* | 0.000 | 0.000 | 0.0002 | |||
| KCC | 0.569 | 0.371 | 0.249 |
SBs enriched with 10% of MLP from all varieties received the lowest score for the sensory attributes, particularly in taste. This could be due to the bitter and astringent taste of the major phenolic compounds contained in mango leaves, such as phenolic acids, xanthones, benzophenones, tannins, and flavonoids [Rasouli et al., 2017].
Texture profile of sliced bread enriched with mango leaf powder
The texture parameters of SBs enriched with MLP and control SB are shown in Table 6. The control SB had higher hardness, chewiness, and gumminess values in all breads evaluated. The high hardness of the control bread may be due to not having additional fiber unlike the enriched breads. Fiber absorbing water changes the structure of dough and thus reduces the hardness of bread. Bourekoua et al. [2018] obtained similar results; their control bread presented a greater hardness than these with the Moringa oleifera leaf powder. However, an excess of fiber can have a negative effect on hardness, as seen in the case of SB enriched with 10% of MLP (significantly higher value than for SB enriched with 2% of MLP, Table 6). In this regard, Mau et al. [2020] demonstrated that a relationship must be found between the percentage of potato leaf powder and the amount of water added to the formulation to maintain or improve the texture characteristics of sliced bread.
Table 6
Texture profile of wheat flour sliced breads (SB control) and sliced bread (SB) enriched with leaf powders (replacement of 2%, 6%, and 10% of wheat flour) from three mango varieties (Criollo, Kent and Edward).
As mentioned above, the findings indicate that MLP incorporation reduced the hardness of SB when compared to the control SB. However, increasing the amount of MLP had a directly proportional effect on the hardness of the tested breads. Khan et al. [2023] observed an inverse relationship; hardness and springiness of wheat flour leavened bread significantly decreased with rises in supplementation levels of Moringa oleifera leaf powder. However, gumminess exhibited a slightly increasing tendency as compared to that of control. According to Cao et al. [2023], hardness, chewiness, and gumminess adversely affect bread quality, while springiness and cohesion improve it. These traits were noted in all SBs enriched with 10% of MLP and the control SB. In contrast, the SB enriched with 2% and 6% of MLP from Criollo and Kent varieties provided the best texture profile, as confirmed by sensory evaluations.
A greater springiness index indicates the freshness and elasticity of bread, as it shows the sample’s capacity to revert to its initial shape after being stretched; qualities generally valued by consumers [Cornejo & Rosell, 2015]. In this context, the samples with the highest springiness index included SB Kent 2%, followed by SB Edward at 2% and 6%, as well as SB Kent at 2% and 6% formulations (Table 6).
CONCLUSIONS
Mango leaf powder contained high levels of phenolic compounds and plant pigments and had high antioxidant capacity. Therefore, the partial replacement of wheat flour with MLP (2%, 6%, and 10%) in the preparation of sliced bread improved its total phenolic content, total flavonoid content and antioxidant capacity. The enrichment of bread with MLP modified the chromatic parameters, and the total color difference from the sliced control bread increased with the increase in MLP percentage in the bread recipe. Sliced bread enriched with 2% of mango leaf powder achieved higher scores according to the sensory panel. In summary, mango leaf powder has the potential to be used in the preparation of sliced bread and other products derived from the baking industry, since it can enrich them in phenolic compounds and improve their antioxidant capacity and their sensory attributes of color, taste, and odor.