INTRODUCTION
Antioxidant compounds, such as flavonoids, phenolic acids, carotenoids, and tocopherols, are heavily used in the food, cosmetic, and pharmaceutical industries because of their therapeutic and disease-preventing properties, namely their antimicrobial, anticarcinogenic, antidiabetic, antihypertensive and antiinflammatory activities [Cilek et al., 2012]. They are known to prevent or reduce oxidation, mitigate the adverse effects of free radicals in tissues, and prolong the shelf life of other products and bioactive molecules [Yağmur & Şahin, 2020]. Natural antioxidants can be extracted from plants, for example cranberries and pomegranates. Cranberry extract is highly ranked for its antioxidant quality and quantity [Niesen et al., 2022; Tsirigotis-Maniecka, 2020]. Its antioxidant activity is attributed to the interdependent actions of its organic acids, carbohydrates, and flavonoids. Among the many benefits of cranberry extract, some of the most notable include its ability to inhibit oxidative processes and growth of several tumor cells, and to aid in preventing urinary tract infections by inhibiting adhesion of Escherichia coli to urinary tract tissues [Tsirigotis-Maniecka, 2020].
Pomegranates are another natural source of antioxidants, specifically flavonoids, condensed tannins, ellagitannins, and gallotannins [Shirode et al., 2015]. The peels and seeds of this fruit contain most of these antioxidant molecules but are often discarded during the juice production process [Banerjee et al., 2017]. Specifically, pomegranate peels contain more than 30% of the total anthocyanins in the fruit [Azarpazhooh et al., 2019] and the highest content of punicalagin – a water-soluble ellagitannin. When hydrolyzed in the small intestine, punicalagin has been found to exhibit antioxidant, antifungal, and antibacterial properties [Kaderides et al., 2020]. When recovered, pomegranate peel extracts have also been successfully used as natural antimicrobial agents against Staphylococcus aureus, Escherichia coli, Listeria monocytogenes and Yersinia enterocolitica and have been also proven to elicit beneficial effects against urinary tract infections [Cui et al., 2020]. Furthermore, pomegranate extracts, including peels, can be used in the cosmetic and pharmaceutical industry as therapeutic agents against a broad range of conditions, such as skin inflammation, acne, psoriasis or even skin cancer [Kalouta et al., 2020].
Despite these many benefits, antioxidant-containing fruit extracts have also been found to be very sensitive to environmental conditions, like oxygen, pH, ultraviolet (UV) light, temperature, and humidity. To circumvent these limitations, various protection methods have been used, of which, encapsulation is one of the most common [Kaderides et al., 2019]. By encapsulating bioactive molecules, they can be shielded from the environments that act to degrade them. However, not all encapsulating materials or techniques provide the same degree of protection. The type of encapsulating material and encapsulating technique can profoundly impact the lifetime and stability of bioactive molecules.
Amongst the encapsulation techniques, some common ones include spray-drying, freeze-drying, self-assembly of polymers, emulsification, and liposome preparation [Rostami et al., 2019]. Some of these involve critical temperatures (spray-drying) which can degrade certain bioactive molecules. Others require equipment that is very expensive or produces unsatisfactory yields. Electrohydrodynamic (EHD) processing has been of recent interest because it may be performed at ambient conditions and also because its start-up cost is low. EHD processing is a technique in which a polymeric solution is pumped through a circuit and ejected towards a collector. Because of an electrical potential difference between the polymeric solution and the collector, the polymeric solution is rapidly ejected to the collector. The solvent evaporates and, due to strong electrostatic forces, nano-/micro-particles or microfibers are formed [Rostami et al., 2019]. However, EHD processing is limited by low throughput. Typical flow rates for particle formation are less than 1mL/h, which hinders its application in the industry [Perez-Masia et al., 2015]. This limitation has been addressed by many researchers [Vass et al., 2020] but proposed solutions mostly apply to the industrial space. A new method that can be easily used in lab as well as industrial settings and that drastically improves the throughput is electro-blow spinning (EBS). EBS is a form of EHD processing improved by involving two driving forces during material production instead of one: electric force and air force. This combination enhances productivity, stabilizes the production process, and can also improve properties of the resulting products. With high solution feed rates, EBS is expected to be an important method for mass production of micro- and nano -materials. The EBS technique has already been successfully used to create fibers from polytetrafluoroethylene, hyaluronic acid, or oxide ceramic fibers [Zhou et al., 2017], but, to the authors’ knowledge, it has not been used for microparticle formation.
To better understand how the broad space of particle formation may benefit from EBS, equipment costs, energy costs, and amounts of time needed were compared for three common microparticle formation techniques: freeze-drying, electrospinning, and electro-blow spinning. Comparing first equipment costs, the lab-scale equipment for freeze-drying cost starts from around 15,000 €. There are no commercial set ups for electro-blow spinning, but equipment prepared in-house can cost around 3,600 €. Similarly efficient electrospinning set ups can be assembled for around 3,500 €. Therefore, these numbers suggest that the EBS technique has economic advantages compared to other techniques. Comparing next the energy consumption during microparticles production, we find that the production of the same amount of material (around 15 g of dry material) costs 25 €, 2.5 € and 6 €, for freeze-drying, electrospinning, and electro-blow spinning, respectively. Lastly, when comparing the time required to produce similar amounts of material, EBS consumes around 8 h, while freeze-drying and electrospinning need around 24 and 500 h, respectively. Hence, electro-blow spinning is a much less time-consuming technique.
Of the biomaterials for encapsulation, some of the most widely used are zein, gelatin, whey protein concentrate, silk, collagen, and pullulan [Rostami et al., 2019]. Pullulan is an extracellular, linear polysaccharide produced by the dimorphic fungus Aureobasidium pullulans in starch and sugar crops [Yang et al., 2020]. It is not only used in its pure form for encapsulation, but also to facilitate the ease of use of other biopolymers for encapsulation [Aguilar-Vázquez et al., 2018]. It is a very desirable encapsulating material for tissue engineering and drug delivery for the following reasons: it is non-toxic, odorless, tasteless, and edible; it is easily modified via its backbone of hydroxyl groups; and accepted by the American Food and Drug Administration (FDA) [Carvalho et al., 2020].
One material that, to the authors’ knowledge, has not yet been studied for the encapsulation of bioactive molecules is cricket flour. It is a flour made by dehydrating or roasting insects followed by grinding them into a fine powder. This means that processed cricket flour could be used to formulate supplement food protein powders [David-Birman et al., 2018]. Though crickets and other insects have been slow to disseminate into the Western consumption culture, they offer many great nutritional and environmental benefits. Nutritionally, insects are not only rich in essential amino acids, but also valuable sources of unsaturated fatty acids, dietary fiber, vitamins, and minerals [Mlček et al., 2019; Montowska et al., 2019; Orkusz, 2021]. Furthermore, they are naturally gluten-free, making them desirable for the production of foods for celiac patients [Wieczorek et al., 2022]. Studies have also shown that cultivating crickets with diets equal in quality to that of traditional livestock, results in a food conversion ratio that is about twice as high as that achieved in broiler chicken and pig production [Zielińska et al., 2018]. From an environmental standpoint, cricket farming has been found to produce 80% less greenhouse gas emissions than cattle. Crickets thus pose great potential for use in the food and pharmaceutical industries looking to improve their nutritional and environmental benefits. Presenting them in the form of flour or an encapsulating material may make them more readily accepted by Western consumption culture. Moreover, owing to a high protein content, its good solubility and capacity to form gels [Ndiritu et al., 2019; Stone et al., 2019], cricket flour can be considered as a potential encapsulating material of bioactive compounds.
Protection of bioactive materials from harsh environmental conditions is one of the most important topics for the extension of their shelf life and activity over long storage times. Expanding upon the technologies and materials available for prolonging bioactive viability can contribute to ways of improving human health and quality of life. Furthermore, the possibility of protecting bioactive molecules with materials that have an increased nutritional and environmental value is crucial for proper development. Cricket flour and other food products that can be categorized as superfoods are of high interest in this respect. Therefore, this work has many aims surrounding the protection of bioactive molecules from harsh environmental conditions. Firstly, cricket flour was evaluated as a novel encapsulating material for the protection of cranberry fruit and pomegranate peel extracts. Additionally, considering the many advantages of the EBS technique, EBS was extended for particle production and compared to the commonly used freeze-drying technique.
MATERIALS AND METHODS
Materials and reagents
Pullulan was purchased from Hayashibara Co., Ltd. (Okayama, Japan). Cricket flour, made from finely milled crickets (Acheta domesticus) was purchased from Crunchy Critters (Derby, UK). Cranberry extract (Vaccinium macrocarpon fruit extract) and pomegranate peel extract (Punica granatum peel extract) were purchased from Zrób Sobie Krem (Prochowice, Poland). Distilled and deionized water was used as the solvent for all formulations.
Preparation of pure biopolymers and extract-containing biopolymer solutions
All solutions were prepared at room conditions, in glass bottles and were processed directly after preparation. Cranberry fruit and pomegranate peel extracts and biopolymers (cricket flour and pullulan) were used without further purification. First, solutions of each extract and biopolymer were prepared. Fruit extract solutions were prepared by dissolving in water separately pomegranate peel extract and cranberry extract at the concentration of 25 g/L. To prepare the solution of pullulan (PU), 50 g/L of this biopolymer was dissolved in water. For the preparation of the pullulan-cricket flour blends (PU-CFF), first 100 g/L of cricket flour was suspended in water under ambient conditions. After 2 h of stirring, the mixture was filtrated through lab cellulose filter with thickness of 0.22 mm for removing coarse sediments. The filtrate was dried to determine the dry weight (DW) of cricket flour that passed through the filter. The total solid content of cricket flour filtrate (CFF) was 20 g/L. This filtration allowed for insect debris to be removed so that only the water-soluble compounds, or small dispersed particles which would not affect electro-blow spinning process, remained in the aqueous solution. After this dissolution and filtration of cricket flours, 50 g/L of pullulan was added to the filtrate and dissolved at ambient conditions. For preparation of CFF sample, no additional steps were added. Water solution of CFF was used at a concentration of 20 g/L.
For the preparation of fruit extract-containing pullulan particles, 25 g/L of cranberry or pomegranate extracts were added to pullulan water solutions (at concentration of 50 g/L) and mixed at ambient conditions for about 1 h until completely incorporated. These pullulan-cranberry (PU-Cranberry) and pullulan-pomegranate (PU-Pomegranate) solutions were later processed via electro-blow spinning (as described in a subsequent section) or freeze-dried. For the preparation of the pullulan-cricket flour blends containing the fruit extracts, also 25 g/L of fruit extracts were added to the water solution of pullulan (50 g/L) and cricket flour filtrate (20 g/L). Pullulan-cricket flour filtrate-cranberry (PU-CFF-Cranberry) and pullulan-cricket flour filtrate-pomegranate (PU-CFF-Pomegranate) solutions were prepared acc. to this procedure and later processed by electro-blow spinning or freeze-dried. Pure cricket flour filtrate microparticles containing the fruit extracts were prepared in the same way as PU-CFF particles, but without adding pullulan.
Chemical composition analysis of biopolymers and fruit extracts
Protein content
Total protein content was measured following the Lowry method with some minor modifications [Benito-González et al., 2019]. Briefly, 1 mL of modified Lowry reagent was well mixed with 0.2 mL of each previously prepared solution and incubated for 10 min at room conditions. After incubation, 0.1 mL of the FolinCiocalteu reagent (mixed with ultrapure water 1:1, v/v) was added and vortexed. Mixed solutions were then incubated for 30 min at room temperature and covered from light. The blank was prepared by mixing 0.2 mL of water with respective amounts of Lowry and Folin-Ciocalteu reagents. After incubation, the absorbance was measured with UV-1800 UV-vis spectrophotometer (Shimadzu, Kyoto, Japan) at 750 nm. The standard curve was prepared with serial dilutions of bovine serum albumin (BSA). Total protein content was expressed as g of proteins per 100 g of the analyzed dry material. Determination was carried out in triplicate.
Lipid content
Total lipid content was determined following the sulpho-phospho-vanillin method, with some minor modifications [Benito-González et al., 2019; Frings & Dunn, 1970]. A phospho-vanillin reagent was prepared by dissolving vanillin in water at a concentration of 6 g/L. A portion of 350 mL of the vanillin solution was then mixed with 50 mL of water and 600 mL of concentrated phosphoric acid. For sample analysis, 20 µL of each previously prepared material solution was mixed with 200 µL of concentrated sulphuric acid, well stirred, and incubated in boiling water for 10 min. After conditioning samples in cold water for 5 min, 10 mL of the phospho-vanillin reagent were added, mixed, and the mixture was incubated at 37°C for 15 min. A blank was prepared with 20 µL of 96% ethanol and respective amounts of reagents. The absorbance was measured at 540 nm using a UV-1800 UV-vis spectrophotometer (Shimadzu, Kyoto, Japan). A calibration curve was plotted using known concentrations of sunflower oil, and total lipid content was expressed as g of lipids per 100 g of the analyzed dry material. Determination was carried out in triplicate.
Characterization of physical properties of biopolymer and extract-containing biopolymer solutions
All dynamic viscosity measurements were taken at 20°C and atmospheric pressure using the Rotavisc – rotational viscometer (IKA, Königswinter, Germany) equipped with a low-viscosity adapter. Conductivity measurements were performed with a multifunction device (CX-705, Elmetron, Zabrze, Poland) equipped with a conductivity electrode (ECF-1, Elmetron, Zabrze, Poland). Surface tension was measured following the Wilhemy plate method using an EasyDyne K20 tensiometer (Krüss GmbH, Hamburg, Germany). All physical property determinations for PU, PU-Cranberry, PU-Pomegranate, CFF, CFF-Cranberry, CFF-Pomegranate, PU-CFF, PU-CFF-Cranberry and PU-CFF-Pomgranate solutions were carried out in triplicate.
Preparation of biopolymer encapsulated extracts
Electro-blow spinning
Electro-blow spinning (EBS) equipment was assembled in-house and used for the encapsulation of pomegranate and cranberry extracts with PU, PU-CFF and CFF. The EBS apparatus consisted of a feeding system, 0–30 kV power supply (Acopian, Easton, PA, USA), air flow system (air compressor) (Airpress, Przeźmierowo, Poland), and grounded collector equipped with lab cyclones, where all produced materials were collected. The anode was connected to a coaxial needle system where the 0.8 mm internal needle was connected to a syringe containing the sample solution and the 1.0 mm external needle was connected to an air flow system. The processing of each extract-containing biopolymer solution was performed in room conditions. Dried particles (PU-Cranberry-EBS, PU-Pomegranate-EBS, PU-CFF-Cranberry-EBS, PU-CFF-Pomegranate-EBS, CFF-Cranberry-EBS and CFF-Pomegranate-EBS) were collected to glass vials and stored in them under 0% of relative humidity (RH) and temperature of 4°C (enclosed in a desiccator with silica gel which was left in the fridge). An air compressor with maximum air compression up to 800 kPa was used to create air pressure that dried the formed particles and helped them reach the collector. All samples were prepared under the same air pressure (200 kPa). Flow rates ranged from 15 to 17 mL/h, and the applied voltage varied from 13 to 17 kV (Table 1). In one working cycle, ca. 20 g of the material were obtained from 300 mL of the used solution.
Table 1
Electro-blow spinning (EBS) processing parameters of pullulan (PU), cricket flour filtrate (CFF) and pullulan-cricket flour filtrate (PU-CFF) polymers that were used to encapsulate cranberry juice and pomegranate peel extracts.
Freeze-drying
The freeze-drying (FD) technology was used to compare encapsulation technologies as well as to quantify and compare extract stability in the PU, CFF and PU-CFF blend. Before freeze-drying, extract-containing biopolymer solutions were frozen at −80°C for 24 h, and then placed into a VirTis Genesis 35 EL freeze-dryer (SP Scientifics, Warminster, PA, USA) at the pressure of 10 Pa for the next 24 h. The obtained powders (PU-Cranberry-FD, PU-Pomegranate-FD PU-CFF-Cranberry-FD, PU-CFF-Pomegranate-FD, CFF-Cranberry-FD, CFF-Pomegranate-FD) were stored at 0% RH and 4°C, enclosed in a desiccator with silica gel and left in the fridge until use.
Encapsulation efficiency evaluation
The amount of cranberry or pomegranate extract incorporated into pullulan, pullulan-cricket flour filtrate and cricket flour filtrate particles was analyzed by UV-vis spectroscopy according to a protocol adapted from the article by Alehosseini et al. [2019]. Specifically, all materials were dissolved in ultra-pure water (20 g/L), and the absorbance was measured at 280 and 292 nm for solutions of microparticles with cranberry fruit and pomegranate peel extracts, respectively, using a UV-1800 UV-vis spectrophotometer (Shimadzu, Kyoto, Japan). Calibration curves for each extract were obtained by tracking the absorbance of varying concentrations of the pure extracts at 280 and 292 nm (R2>0.995). These curves were used to determine the total encapsulated fruit extract. Encapsulating materials produced no peaks around 280 and 292 nm, confirming that they did not contribute to absorbance values. Theoretical encapsulated fruit extract was taken as the amount of extract present in the solution before EBS or FD. Encapsulation efficiency (EE) was then calculated using Equation (1) with analysis of three independent replicates of each sample.
Moisture content determination
To estimate moisture content of the extracts and microcapsules, all materials (ca. 0.5 g) were placed in a lab dryer and dried at 110°C. The drying process stopped when the weight of tested materials attained constant [Hamdan et al., 2020]. Measurements were performed in triplicate.
Morphological and size distribution characterization of materials formed via EBS
Scanning electron microscopy (SEM) was conducted using an S-4800 microscope (Hitachi, Tokyo, Japan) with an accelerating voltage of 10 kV for SEM and a working distance of 40 mm. Materials created via EBS analyzed by SEM, were not sputtered with any conductive coating. Particle size diameters of all materials were measured by means of ImageJ software (version 1.52n, NIH & LOCI, University of Wisconsin, Madison, WI, USA) from the SEM micrographs in their original magnification [Çanga & Dudak, 2019; Saud et al., 2023]. In all cases, a minimum of 150 particles were analyzed to determine their size distribution (or average sizes).
Infrared spectroscopy analysis
Fourier transform infrared spectra (FTIR) were collected for the chemical characterization of pure biopolymers, pure and encapsulated via EBS and FD fruit extracts by using the attenuated total reflection (ATR) attachment on Invenio S FT-IR (Bruker, Billerica, MA, USA) spectrometer. Single spectra were averaged over 24 scans at 4 cm−1 resolution in the wavelength range from 400 to 4,000 cm−1. All analyses were performed in duplicate.
Total phenolic content determination
Total phenolic content was analyzed using the method with a Folin-Ciocalteu reagent, according to a method adapted from Cilek et al. [2012]. To 0.5 mL of pure and encapsulated in biopolymers fruit extract solutions (1 mg/g), 2.5 mL of the Folin-Ciocalteu reagent (10 times diluted with water) were added and, followed by 2 mL of Na2CO3 (75 g/L). The sample was incubated for 5 min at 50°C and then cooled. For a control sample, 0.5 mL of distilled water was used. The absorbance was measured at 760 nm. The results were expressed in g of gallic acid equivalent per g of dry weight of sample (mg GAE/g DW). Three-replicate analyses were performed for each type of material.
Antioxidant activity analysis
ABTS radical cation scavenging activity
Antioxidant activity of the fruit extracts and microparticles, and changes of this activity after UV light radiation were analyzed by an improved 2,2-azino-di-(3-ethylbenzothiazoline-sulphonic acid radical cation (ABTS•+) method as described by Re et al. [1999] and Qabaha et al. [2019]. In brief, the ABTS•+ stock solution (7 mM) was prepared through the reaction of 7 mM ABTS and 2.45 mM of potassium persulphate as the oxidant agent. The working solution of ABTS•+ was obtained by diluting the stock solution in phosphate-buffered saline (PBS) to produce an absorbance of 0.70±0.02 at λ=734 nm. Samples were dissolved in water at a concentration of 1 mg/mL, and 100 µL of the solution was added to 900 µL of the ABTS•+ solution. Absorbance readings at wavenumber of 405 nm were taken at room conditions exactly 10 min after initial mixing. A calibration curve was plotted by using 6-hydroxy-2,5,7,8-tetra-methylchromane-2-carboxylic acid (Trolox). The antioxidant activity of pure and encapsulated cranberry and pomegranate fruit extracts was expressed as µmol Trolox equivalents (TE) per g sample DW. Determination was carried out in triplicate.
DPPH radical scavenging activity
DPPH radical scavenging activity was determined following the method described by Okonogi et al. [2007]. The tested pure and encapsulated in biopolymer fruit extracts were mixed with ethanol to prepare the ethanolic test solution (1 mg/mL). DPPH• (100 μM) was dissolved in ethanol and mixed with an aliquot of 100 μL of the sample solution. After vigorous shaking, the mixtures were left to stand for 30 min in the dark at room temperature. Then, the absorbance was measured at 517 nm. Trolox was used as a standard, and the results were expressed as mmol TE/g sample DW.
Accelerated degradation studies
To accelerate the oxidation of fruit extracts and more rapidly evaluate the protecting effect provided by the biopolymers, all produced microparticles were exposed to UV light radiation for a time span of 30 h, using an Ultra-Vitalux lamp (OSRAM Licht AG, Munich, Germany). This lamp operates with a power of 300 W that is generated by a quartz discharge tube and a tungsten filament. As described by the manufacturer, it produces a blend of radiation very similar to that of natural sunlight where the radiation of 315−400 nm after 1 h of exposure is of 13.6 W, and the radiation of 280−315 nm after 1 h of exposure is of 3.0 W. Sample stability was determined using a UV-1800 UV-vis spectrophotometer (Shimadzu, Kyoto, Japan) after various UV light exposure times (from 0 to 30 h). The absorbance was measured in the wavenumber range from 220 to 700 nm. All measurements were performed in triplicate.
RESULTS AND DISCUSSION
Chemical composition of encapsulating and bioactive materials
In this study, protein, and lipid contents were analyzed in pullulan (PU), cricket-flour filtrate (CFF), and pullulan-cricket flour filtrate (PU-CFF), and respective results are presented in Table 2. Additional cricket flour information from the provider reveals that it contained 56.8 g of proteins, 29.3 g of lipids and only 5.5 g of carbohydrates in 100 g of flour. Because of the very low carbohydrate content in cricket flour (according to provider’s information and already published data [Montowska et al., 2019; Orkusz, 2021]) and the fact that pullulan consists of 99.9% maltotriose, carbohydrate composition was not analyzed for these materials.
Table 2
Total protein and lipid content of extracts and encapsulating materials including pullulan (PU), cricket flour filtrate (CFF) and pullulan with cricket flour filtrate (PU-CFF).
Data presented in Table 2 show that CFF and PU-CFF both contained a comparably high protein content. Though being a highly pure maltotriose-chain polymer, pullulan was still found to contain residual amounts of proteins. However, this protein content was 10 times smaller than CFF. The slightly higher protein content in PU-CFF compared to CFF was attributed to this small amount of protein in PU. The protein content determined from cricket flour filtrate was lower than that provided by the supplier for cricket flour. This fact can be explained by material loss during filtration. Despite this, literature data provide protein content in cricket powders to range from 42 to 45 g/100 g [Montowska et al., 2019], which represent similar values to data presented in this research.
The protein content in pomegranate peel extract and cranberry fruit extract was 2.87 and 3.77 g/100 g, respectively. These contents were similar to the fruit extracts providers’ information. Moreover, a high content of lipids was not detected in any of the analyzed fruit extracts, which also coincides with the information from the provider.
In the case of pullulan, small contents of protein and lipids were detected. This was probably an effect of the presence of some residuals after polymer purification from microorganisms producing this material. The presence of lipids in materials with cricket flour filtrate was observed at levels of approximately 19 g/100 g for pure CFF and 21 g/100 g for CFF mixed with PU. Total lipid content was lower when compared to provider’s information, but similar to data presented by another scientific group for cricket powder – around 25 g/100 g [Montowska et al., 2019].
This information suggests that the main encapsulating macromolecules of PU were polysaccharides and that lipids and proteins which for CFF and PU-CFF were dominating encapsulating materials, were residual molecules in the encapsulation process.
Physical properties of biopolymer and extract-containing biopolymer solutions
The conductivity, surface tension and dynamic viscosity of the solutions of biopolymers and biopolymers with pomegranate peel and cranberry fruit extracts are shown in Table 3. All solutions presented conductivity in the range of 0.04–2.34 mS/cm. Pure PU solution showed the lowest values of conductivity of 0.04 mS/cm, which was similar to already published data [Tomasula et al., 2016]. When cricket flour filtrate was added to the pullulan solutions, the conductivity increased up to 1.21 mS/cm, suggesting that the filtered cricket flour contributed by increasing the concentration of ions in the solution. This result was not surprising as the protein content of the CFF was significant, and proteins are charged macromolecules with high conductivity. Similar conductivity behaviors of solutions containing proteins (like casein solutions) have been previously observed [Tomasula et al., 2016]. Moreover, the conductivity increased when fruit extracts were added to the solutions (Table 3), which was also likely an effect of an increased ion concentration coming from polyphenols of the extract solutions.
Table 3
Physical properties of solutions of cranberry fruit extract and pomegranate peel extract mixed with encapsulating materials including pullulan (PU), cricket flour filtrate (CFF) and pullulan with cricket flour filtrate (PU-CFF).
Surface tension of the solutions ranged from 33.18 to 37.23 mN/m. The addition of extracts caused a slight reduction in its value, which was significant (p<0.05) in the solutions with CFF and pure pullulan as encapsulating materials. Similar reductions have been reported previously [Torres-Giner et al., 2017], where mixing biopolymers with Aloe vera extract caused slight changes in solution’s surface tension. The dynamic viscosities of the solutions varied with values between 11.59 and 42.11 cP×s when fruit extracts were added to biopolymer solutions. Much higher ranges of viscosity were determined in the solutions where pullulan was added as the encapsulating material. When CFF was used to protect fruit extracts, solution viscosities presented 4 times lower dynamic viscosity range. It should be noted that all analyzed physical properties of the prepared solutions where typical for solutions that have been used for various encapsulating techniques, e.g., electro-spinning, blow-spinning, and spray-drying. For the electrospinning technique, solution conductivity should not increase above 500 mS/cm [Ramakrishna, 2007]. Best surface tension and conductivity values for successful solution electrospinning are 20-45 mN/m and 170-21,500 cP×s, respectively [Williams et al., 2018]. Moreover, for electrospraying, solution surface tension and conductivity should be similar, as in the case of electrospinning, but dynamic viscosity should not be lower than 2 cP×s to successfully obtain microparticles [Zhang et al., 2019].
Encapsulation efficiency and moisture content of microparticles
Encapsulation of pomegranate peel and cranberry fruit extracts was carried out using EBS and FD methods. The processing conditions for the in-house electro-blow spinning of PU, CFF, PU-CFF solutions and extract-containing biopolymer solutions were optimized in a preliminary study and are presented in Table 1. The most effective material drying was achieved when the working distance was set up at 100 cm. In the case of cricket flour filtrate (CFF), smaller working distance had to be used, up to 80 cm, because with longer distances, the material did not reach the collector but instead spread over the working space. In the case of PU-CFF-Cranberry and PU-CFF-Pomegranate samples, the working distance was set up to 120 cm. When a shorter distance was used, the material reaching the collector was still wet. This could probably be caused by the higher concentration of the encapsulating material used to protect fruit extracts and the need for greater distance to evaporate all the water. A slightly higher voltage was needed for particle formation for the samples with higher conductivity and dynamic viscosity ranges of the used solutions. The same situation was also observed in the case of electrospinning method applied for particle formation [Ramakrishna, 2007].
EE results of all analyzed samples are presented in Table 4. Obtaining such a high EE for microcapsules made with the EBS technique (above 80% for PU blends and around 60% where CFF was used as an encapsulating matrix in comparison to 51–79% EE for the samples made by FD) supported that the constructed EBS equipment was working properly and allowed for effective encapsulation of fruit extracts in all biopolymers. Moreover, EE values obtained for the freeze-dried materials were in line with findings from previous research, where EF of fish oil encapsulation in whey protein concentrate via FD and EE was about 63% [El Ghannam et al., 2015]. In the case of the EBS technique, EE values were similar to those obtained through other techniques such as spray-drying or electro-spinning For example, saffron encapsulated in gelatin via electro-spinning presented EE at 63–74 % and via freeze-drying – at 51–65% [Golpira et al., 2021].
Table 4
Encapsulation efficiency (EE) of cranberry juice and pomegranate peel extracts in pullulan (PU), cricket flour filtrate (CFF) and pullulan-cricket flour filtrate (PU-CFF) by electro-blow spinning (EBS) and freeze-drying (FD) methods and moisture content (MC) of obtained microparticles.
Moisture content of all produced materials is presented in Table 4. All samples made via EBS or FD contained residual amount of water after processing. The lowest water content was observed in CFF-Cranberry sample made via EBS. Moisture content of the other materials produced via EBS varied from 2.3 up to 2.7 g/100 g, and there were no significant differences between them in this respect. In the case of the samples made via FD, the moisture content was significantly higher in PU-Cranberry (3.2 g/100 g), PU-Pomegranate (3.2 g/100 g), and PU-CFF-Pomegranate (3.1 g/100 g). These data were similar to those presented for beads of blueberry juice encapsulated in maltodextrins via FD [Wilkowska et al., 2016].
Morphological characteristics of electro-blow spun particles
The particles obtained via electro-blow spinning (EBS) were characterized with scanning electron microscopy (SEM). Figures 1, 2, and 3 display representative micrographs of the various particles without extract, with cranberry extract, and with pomegranate extract. These micrographs prove the successful particle formation with the EBS technique.
Figure 1
Scanning electron microscopy (SEM) micrographs of pure pullulan microparticles, PU-EBS (A), cranberry fruit extract encapsulated in pullulan, PU-Cranberry-EBS (B) and pomegranate peel extract encapsulated in pullulan, PU-Pomegranate-EBS (C), as well as microparticle size diameter distribution of PU-EBS (D), PU-Cranberry-EBS (E) and PU-Pomegranate-EBS (F).

Figure 2
Scanning electron microscopy (SEM) micrographs of pure pullulan-cricket flour filtrate microparticles, PU-CFF-EBS (A), cranberry fruit extract encapsulated in pullulan-cricket flour filtrate, PU-CFF-Cranberry-EBS (B) and pomegranate peel extract encapsulated in pullulan-cricket flour filtrate, PU-CFF-Pomegranate-EBS (C), as well as microparticle size diameter distribution of PU-CFF-EBS (D), PU-CFF-Cranberry-EBS (E) and PU-CFF-Pomegranate-EBS (F).

Figure 3
Scanning electron microscopy (SEM) micrographs of pure cricket flour filtrate microparticles, CFF-EBS (A), cranberry fruit extract encapsulated in cricket flour filtrate, CFF-Cranberry-EBS (B) and pomegranate peel extract encapsulated in cricket flour filtrate, CFF-Pomegranate-EBS (C), as well as microparticle size diameter distribution of CFF-EBS (D), CFF-Cranberry-EBS (E) and CFF-Pomegranate-EBS (F).

EBS-processed pullulan particles were relatively homogenous, smooth, and round (Figure 1A). No fiber structures were observed. Mean microparticle size was about 3.5 µm, and about 80% of all microparticles had diameters that were less than 4 µm (Figure 1D). When encapsulating fruit extracts, the structure, shape, and size of the pullulan microparticles varied depending on the chosen extract. In the case of PU-Cranberry-EBS, very tiny fiber structures were observed in combination with round, diskshape with concave center microparticles (Figure 1B). Mean microparticle size of PU-Cranberry-EBS was about 5.6 µm, and more than 70% of them presented sizes below 6 µm (Figure 1E). For this bioactive-biopolymer combination, microparticles smaller than 1 µm were not observed. In the micrograph of PU with pomegranate extract (PU-Pomegranate-EBS), no fiber-structures were observed (Figure 1C). The resulting EBS microparticles of this material were like the ones made from PU-Cranberry material. Mean microparticle size diameter was about 5.3 µm, with almost 80% of the microparticles having sizes lower than 6 µm (Figure 1F).
When pullulan with cricket flour filtrate were mixed and used as the encapsulating material through EBS technology, the resulting microparticles were slightly larger than those made with pure PU material. Specifically, the average PU-CFF-EBS microparticle diameter was around 6.2 µm and no microparticles smaller than 2 µm were observed (Figure 2D). In addition to the microparticles, some very tiny fiber structures were observed. With cranberry extract, PU-CFF-Cranberry-EBS microparticles were like pure PU-CFF-EBS microparticles in terms of size and shape (Figure 2B and 2E). With pomegranate extract (PU-CFF-Pomegranate-EBS), the mean microparticle size was about 5.9 µm with the majority (around 80%) of the produced microparticles being less than 8 µm in diameter (Figure 2C and 2F).
In the case of cricket flour filtrate materials processed by EBS, round and slightly deformed microparticles were observed, where the mean size diameter was 3.94 µm, 4.9 µm and 4.6 µm for pure cricket flour filtrate, CFF microparticles loaded with cranberry extract and CFF microparticles loaded with pomegranate extract, respectively (Figure 3). About 70% of the microparticles produced from CFF were less than 10 µm (Figure 3D).
When analyzing microparticle size diameter for all the materials produced with the EBS technology, 90% of the microparticles presented size less than 9 µm for PU-EBS and PU-Pomegranate-EBS samples. The widest microparticle size distribution was observed for the pure cricket flour filtrate and CFF-Pomegranate-EBS samples. Only 55% and 65% of those samples, respectively, presented size distribution lesser than 9 µm. When dealing with a polymer solution with low electrical conductivity, the electrostatic force has two components: normal and tangential to the surface of the drop. The effect of these forces on a droplet of polymer solution and on the coaxial stretching of the stream of material is the formation of various forms of nanomaterials and it could affect the material size and shape [Ramakrishna, 2007].
Considering an effect of solution dynamic viscosity on the final material shape and size, we can observe that with increasing viscosity, the mean size of microparticles obtained with EBS also increased. With lower solution dynamic viscosity, materials made by EHD form mostly microparticles and with increasing viscosity the solution can form fibers instead of microparticles [Ramakrishna, 2007]. This solution behavior was observed in all solutions formed with pullulan, where dynamic viscosity varied between 41 and 43 cP×s. Mean microparticle size of the materials made with higher viscosity numbers was greater, and the materials tended to form microparticles with some tiny fibers between them. Moreover, solutions with lower dynamic viscosity, like the materials formed from cricket flour without pullulan, displayed smaller mean microparticle size. This observation can be explained by distribution of forces that affect a solution droplet leaving an injector. A greater distribution of forces is needed to stretch a more viscous solution and, conversely, less forces are needed to stretch less viscous solutions; thus, the obtained materials’ size can be smaller [Ramakrishna, 2007].
FTIR spectra characteristics of biopolymers, fruit extracts and extract-containing biopolymer microparticles
All materials made from biopolymers containing pomegranate and cranberry extracts, as well as the pure biopolymers, were characterized using FTIR spectroscopy. These spectra are shown in Figure 4 and 5. Characteristic peaks of the spectra for the different encapsulated materials (typical for functional groups of polysaccharides, proteins, or lipids) were chosen to highlight similarities in the chemical composition of the microparticles created with non-processed extracts and to confirm the presence of the extracts in the microparticles.
Figure 4
Fourier transform infrared spectra of cranberry fruit extract, pomegranate peel extract and encapsulating materials: pullulan (PU), pullulan mixed with cricket flour filtrate (PU-CFF) and cricket flour filtrate (CFF).
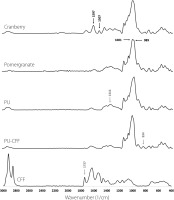
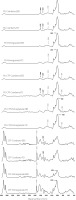
Figure 5
Fourier transform infrared spectra of cranberry fruit extract and pomegranate peel extract encapsulated via electro-blow spinning (EBS) and freeze-drying (FD) in pullulan (PU-Cranberry-EBS, PU-Cranberry-FD, PU-Pomegranate-EBS and PU-Pomegranate-FD), pullulan mixed with cricket flour filtrate (PU-CFF-Cranberry-EBS, PU-CFF-Cranberry-FD, PU-CFF-Pomegranate-EBS and PU-CFF-Pomegranate-FD) and cricket flour filtrate (CFF-Cranberry-EBS, CFF-Cranberry-FD, CFF-Pomegranate-EBS and CFF-Pomegranate-FD).
In the case of FTIR spectra corresponding to both fruit extracts (cranberry and pomegranate), characteristic spectral region for polysaccharides was presented: region 1,500–1,200 cm−1 – including deformational vibrations of groups with local symmetry, like CH2 and C–OH deformations encountered in carbohydrates and region 1,200–800 cm−1 [Wan et al., 2021]. Those typical for polysaccharides regions were also easy to observe on FTIR spectra of pullulan and its mixture with cricket flour filtrate (Figure 4). No characteristic bands from proteins and lipids were observed on the FTIR spectra for either fruit extracts or pullulan spectra. These observations agree with data presented previously, where the total protein and lipid contents in the fruit and biopolymers were analyzed (Table 2).
In the case of cricket flour filtrate spectra, many regions characteristic for fatty acids and proteins were observed (Figure 4), proving the presence of those compounds in the samples, and thereby confirming previously presented data in Table 2. One of the most characteristic peaks detected on the spectra of pure cricket flour filtrate were those at wavenumbers of 2,850 and 2,917 cm−1. Those peaks correspond to C–H stretching vibrations, or more precisely, to the symmetric and antisymmetric (respectively) methylene stretching vibrations in the chemical compounds [Sinclair et al., 1952]. A characteristic peak for fatty acids (1,737 cm−1) was strongly presented in the FTIR spectra of cricket flour filtrate but less present in the spectra of pure fruit extract. This peak corresponds to a strong carbonyl bond [Sinclair et al., 1952].
In the case of fruit extracts, we compared encapsulation in pure pullulan (PU), pullulan-cricket flour filtrate mixtures (PU-CFF) and pure cricket flour (CF) via EBS and FD. When using pure pullulan as the encapsulating material, characteristic peaks for pomegranate and cranberry extracts were observed in FTIR spectra at wavenumbers of 1,001 and 1,507 cm−1, respectively, confirming the presence of extracts in these materials (Figure 5). Additionally, in the FTIR spectra of all analyzed materials, the presence of characteristic regions for carbohydrates were observed at wavenumbers of 1,500–1,200 cm−1 and 1,200–800 cm−1, that correspond to deformational vibrations of CH2, C–OH groups in polysaccharides and monosaccharides, respectively, that come from pullulan. All presented peaks in PU-Cranberry-EBS, PU-Cranberry-FD, PU-Pomegranate-EBS and PU-Pomegranate-FD spectra suggest successful pomegranate extract encapsulation with pullulan via both EBS and FD.
When analyzing the spectra of cranberry and pomegranate extract encapsulated by the mixture of pullulan with cricket flour filtrate (via EBS and FD), characteristic peaks were observed at wavenumbers of 989 and 1,597 cm−1 (Figure 5). These peaks can be observed on spectra corresponding to pomegranate peel extract and cranberry fruit extract, respectively. Peaks at those wavenumbers correspond to strong stretching C=C bonds, suggesting the presence of common polyphenol compounds in the extracts [Hong et al., 2021; Nawrocka et al., 2020]). The presence of those peaks in the spectra of PU-CFF-Cranberry-EBS, PU-CFF-Cranberry-FD, PU-CFF-Pomegranate-EBS and PU-CFF-Pomegranate-FD, but not in the pure encapsulating material spectra (Figure 4), suggests successful encapsulation with both techniques. Moreover, in all analyzed samples we have observed the presence of peaks in regions typical for carbohydrates (1,500–1,200; 1,200– 800 cm−1) and for proteins (1,650–1,550 cm−1) [Hong et al., 2021; Sinclair et al., 1952], which suggests that the dominant encapsulating materials were both carbohydrates and proteins from both the pullulan and cricket flour filtrate.
For the samples of fruit extracts encapsulated with filtered cricket flour by freeze-drying and EBS (Figure 5), peaks were observed in the regions typical for fatty acids (2,950–2,850 cm−1 and 1,800– –1,700 cm−1). These regions correspond to C–H and C=O stretching vibrations, respectively. Another interesting region detected on those spectra occurred at wavenumbers of 1,650–1,500 cm−1, which is typical for the presence of protein, amide I and II region [Barth, 2007]. This suggests that the main encapsulating materials for pomegranate peel extract were proteins and lipids coming from the cricket flour extract. Moreover, in the FTIR spectra of pure extracts and encapsulated materials, peaks were observed in the regions typical for carbohydrates (1,500–200 and 1,200–800 cm−1), which proves the presence of fruit extracts in the samples encapsulated with cricket flour filtrate via EBS and FD.
Stability of electro-blow spun and freeze-dried pure and encapsulated fruit extracts after UV light radiation
Antioxidant activity analyzed via ABTS and DPPH assays and total phenolic content of cranberry fruit and pomegranate peel extracts and microparticles loaded with extracts are presented in Table 5. The total phenolic content and antioxidant activity of microcapsules were around three times lower than those of the extracts. Considering encapsulation efficiency and the encapsulating material-to-extracts ratio, the lower antioxidant activity determined in the case of immobilized pomegranate of cranberry extracts seems to be understood. Moreover, ABTS•+ and DPPH• scavenging activities and the total phenolic content of pomegranate peel extract and cranberry extract were similar to those previously presented in various research [Elfalleh et al., 2009; Fischer et al, 2011; Qabaha et al., 2019; Wang et al., 2023]. Analyses of the total phenolic content and antioxidant activity were also performed with encapsulating materials (data not shown); phenolic content and antioxidant activity were undetectable for biopolymers.
Table 5
Total phenolic content and ABTS•+ and DPPH• scavenging activity of pomegranate peel and cranberry juice extracts in free forms and encapsulated in pullulan (PU), cricket flour filtrate (CFF) and pullulan-cricket flour filtrate (PU-CFF) by electro-blow spinning (EBS) and freeze-drying (FD) methods.
Degradation process of cranberry fruit and pomegranate peel extracts was analyzed via measuring the reduction in normalized absorbance and ABTS•+ scavenging activity as a result of 30 h of UV light radiation (Figures 6 and 7, respectively). For both fruit extracts encapsulated in different biopolymers, significantly higher stability was observed with UV light exposure than for the extracts that were not encapsulated. After 30 h of UV light exposure, unprotected cranberry and pomegranate extracts exhibited a reduction of absorbance at wavelengths of 280 and 292 nm, respectively, by around 53% (Figure 6A) and over 60% (Figure 7A). When comparing absorbance changes data to antioxidant activity reductions after 30 h of UV light radiation, similar behavior of fruit extracts was observed (Figure 6B and 7B). The initial ABTS•+ scavenging activity of unprotected cranberry fruit extract reached 34.91 μmol TE/g (Table 5), which was comparable with data published by other research groups [Baranowska & Bartoszek, 2016]. After 30 h of UV light radiation, the unprotected extract lost up to 50% of its antioxidant activity (Figure 6B). The initial ABTS•+ scavenging activity of pomegranate peel was 40.78 μmol Trolox/g (Table 5) and dropped by more than 55% after 30 h of UV light radiation (Figure 7B).
Figure 6
Reduction in absorbance at a wavelength of 280 nm (A) and reduction of ABTS•+ scavenging activity (B) of pure and encapsulated cranberry fruit extract after 30 h of UV light radiation. Different letters above bars indicate significant differences between the samples (p<0.05). PU, pullulan; CFF, cricket flour filtrate; PU-CFF, pullulan mixed with cricket flour filtrate.
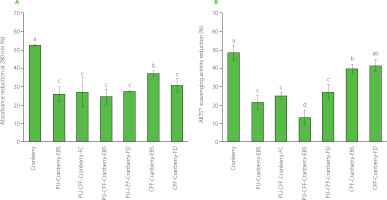
Figure 7
Reduction in absorbance at a wavelength of 292 nm (A) and reduction of ABTS•+ scavenging activity (B) of pure and encapsulated pomegranate peel extract after 30 h of UV light radiation. Different letters above bars indicate significant differences between the samples (p<0.05). PU, pullulan; CFF, cricket flour filtrate; PU-CFF, pullulan mixed with cricket flour filtrate.
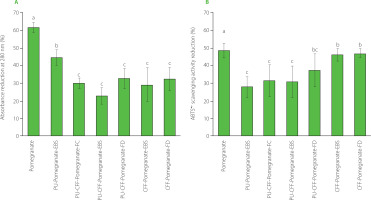
Conversely, the fruit extracts encapsulated in pure pullulan and pullulan with cricket flour filtrate via EBS and FD degraded around 13–25% (cranberry fruit extract) and 25–40% (pomegranate peel extract) after the same UV light exposure (Figure 6A and 7A). The differences in reductions between these samples were statistically not significant (p≥0.05). This can lead to a conclusion that the cranberry fruit extract encapsulated with pullulan and its mixture with a cricket flour extract via both – EBS and FD, exhibited similar protective behavior. Like for the absorbance changes, much smaller antioxidant activity reduction was observed when the fruit extracts were encapsulated with pullulan and its mixture with cricket flour filtrate via EBS and FD (Figure 6B, PU-Cranberry-EBS, PU-Cranberry-FD and PU-CFF-Cranberry-FD; Figure 7B, PU-Pomegranate-EBS, PU-Pomegranate-FD, PU-CFF-Pomegranate-EBS and PU-CFF-Pomegranate-FD). The reduction of ABTS•+ scavenging activity after 30 h of UV light radiation in the case of these samples was about 20–25% and 37–45% for cranberry and pomegranate extracts, respectively, and differences between the obtained data were not statistically significant (p≥0.05). The reduction of ABTS•+ scavenging activity after 30 h of UV light radiation was statistically significant (p<0.05) only in the case of the cranberry fruit extract encapsulated with pullulan mixed with the cricket flour extract via EBS (Figure 6B). In this case, the sample lost only 13% of its antioxidant activity. These results indicate that all encapsulated samples (prepared by both, electro-blow spinning and freeze-drying) present excellent antioxidant protection from UV light. Moreover, adding a filtrate of cricket flour to pullulan can improve protective properties of the polysaccharide itself. Also from these data, electro-blow spinning can be considered to ensure the same efficiency as freeze-drying with respect to encapsulation and protection of bioactive compounds.
To sum up, pullulan itself and mixed with cricket flour filtrate were the best protecting materials for antioxidants in the extracts exposed to UV radiation. It was not possible to explicitly define a single material that proved most successful in protecting the fruit extracts from harsh environmental conditions. We did, however, clearly see differences when comparing data to the materials protected only by cricket flour filtrate. Data obtained from encapsulated fruit extract stability and antioxidant activity analysis suggest that the cricket flour filtrate can still be used as an effective encapsulating material for antioxidant components, but its protective property significantly increases when mixed with polysaccharides from pullulan. Moreover, there was no significant difference in protecting activity, when extracts were encapsulated via electro-blow spinning or freeze-drying. This result indicates the EBS to be an effective and efficient method for bioactive compounds protection from harsh environment conditions and comparable to freeze-drying.
CONCLUSIONS
In summary, two bioactive extracts, namely cranberry fruit extract and pomegranate peel extract, were successfully encapsulated via two methods: electro-blow spinning and freeze-drying. The encapsulating materials used were pullulan, cricket flour filtrate, and a mixture of pullulan and cricket flour filtrate. UV-vis spectroscopy and an ABTS assay demonstrated that encapsulation of the fruit extracts significantly improved extract stability and reduced the loss of antioxidant activity after 30 h of UV light radiation. There were no significant differences in the level of protection when EBS or FD was used as an encapsulating technique or when pullulan and cricket flour were used. Data presented in this paper suggest that cricket flour can be considered as a new encapsulating material. Furthermore, mixing cricket flour with some extracts that present desired properties in food or cosmetic industry (e.g., antioxidant activity) could lead to greater acceptance of this material in the Western market. These findings indicate that electro-blow spinning can be used as an effective method for the protection of bioactive molecules. The greatest advantage of using the EBS technique is its low equipment and consumption cost, compared to other encapsulation techniques.

