INTRODUCTION
Nowadays, as society’s interest in healthy nutrition continues to grow, food producers are actively searching for safe alternatives to synthetic food dyes, which frequently raise concerns regarding potential adverse health effects. Natural plant-based pigments are emerging as highly appealing options, not only for the absence of their harmful effects but also for their valuable health-promoting properties. Betalains, anthocyanins, carotenoids, and chlorophyll represent four essential groups of plant pigments abundantly found in nature, playing a significant role in maintaining an excellent overall state of health [Cai et al., 2005a; Manzoor et al., 2021; Stintzing et al., 2002].
Betalains, constituting a group of secondary metabolites found in plants of the Caryophyllales order, exhibit structural diversity, enabling their categorization into two groups: red-violet betacyanins and yellow-orange betaxanthins [Wybraniec et al., 2010]. They exert a significant influence on human health due to their various bioactivities, including antioxidant, antibacterial, anticancer, antiviral, and anti-inflammatory potential [Naseer et al., 2019]. Betacyanins derive from betanidin, an imine adduct of betalamic acid and cyclo-3,4-dihydroxyphenylalanine (cyclo-DOPA). Conversely, betaxanthins can be synthesized through the condensation of amino acids or amines with betalamic acid [Gengatharan et al., 2015]. It is worth highlighting that betaxanthins can function as a valuable dietary source of essential amino acids [Cai et al., 2001].
The only commercial source of betalains is the root of red beet (Beta vulgaris L.), which provides pigments in various shades of red and purple betacyanins as well as yellow and orange betaxanthins [Stintzing et al., 2002]. Currently, there are only few other known sources of betaxanthins, including the roots of yellow varieties of B. vulgaris [Spórna-Kucab et al., 2023], flowers of Portulaca grandiflora Hook. [Spórna-Kucab et al., 2022], fruits of Hylocereus polyrhizus [Wybraniec et al., 2009], yellow fruits of Stenocereus pruinosus [Sandate-Flores et al., 2020], yellow pulp of Opuntia ficus-indica [FernándezLópez et al., 2018], tuber skin of Ullucus tuberosus Caldas [Mosquera et al., 2020], pure yellow genotype of Celosia argentea var. plumosa [Cai et al., 2005b], orange-red genotype of Celosia argentea var. cristata [Cai et al., 2005b], and leaves of Amaranthus tricolor L. [Cai et al., 2005b]. However, there are promising alternative sources of betaxanthins that have not been studied yet, such as the leaves of B. vulgaris.
B. vulgaris roots, commonly known as beetroots, rightfully earn the title of a superfood due to their numerous health benefits and their rich content of biologically active compounds, including betaxanthins, betacyanins, carotenoids, and flavonoids [Bangar et al., 2022; Székely & Máté, 2022]. Their bioactive compounds have been demonstrated capable of inhibiting the growth of specific types of cancer cells, scavenging free radicals, mititgating harmful cholesterol levels in the bloodstream, and alleviating inflammation within the body [Bangar et al., 2022; Székely & Máté, 2022]. Undoubtedly, one of the most significant advantages of beetroot is the ease of its cultivation, widespread availability, and affordability. Moreover, beet leaves, often considered as mere waste, are increasingly becoming a staple in our diets, as they constitute a valuable source of the above-mentioned bioactive compounds [Székely & Máté, 2022].
Cacti are ornamental plants with a perennial nature, characterized by succulent stems and slow growth rates. They are known for their exceptional ability to survive in dry conditions, displaying a wide variety of shapes and sizes [Perumal et al., 2019].
Within a year, betalains derived from beetroot are capable of meeting up to 10% of the global demand for food pigments [Manchali et al., 2012; Sadowska-Bartosz & Bartosz, 2021]. The food coloring market is flourishing at an annual growth rate of 4.6%, with estimates projecting it to reach a global market value of 2.3 billion dollars [Prajapati & Jadeja, 2022]. However, cacti, particularly the grafted G. mihanovichii, can also function as a source of betacyanins. Utilizing these plants as an alternative betalain source can broaden the spectrum of colorants and pigments [Belhadj Slimen et al., 2017]. G. mihanovichii grafted cactus has played a notable role in the subtropical regions of South and North America, being responsible for an impressive percentage of cactus production, reaching up to 70% [Belhadj Slimen et al., 2017]. This high percentage constituted a significant portion of exported specimens, primarily focused on the markets of the Netherlands and the United States due to their unique colors and forms, which are highly valued as ornamental potted plants. Currently, they do not have significant applications in the food, medical, or industrial sectors. Nevertheless, it is worth noting that the potential use of this cactus as a source of yellow-orange betaxanthins could greatly expand its export opportunities in the food and medical markets [Manchali et al., 2012; Sadowska-Bartosz & Bartosz, 2021].
The presented research involved the chromatographic analysis of betaxanthin profiles of the leaves of B. vulgaris and grafted cacti of G. mihanovichii, utilizing the high-performance liquid chromatography with a diode array detector coupled to electrospray ionisation mass spectrometry (HPLC-DAD-ESI-MS) technique. The main objective of this study was to investigate alternative natural sources of food colorants, betaxanthins. It is important to note that similar studies have not been conducted to date. Previous investigations primarily focused on the betaxanthin profiles of Beta vulgaris L., Chenopodium formosanum, Opuntia ficus-indica L., and Portulaca grandiflora Hook. [Gamba et al., 2021; Kugler et al., 2004; Otálora et al., 2020; Spórna-Kucab et al., 2023, 2022; Xie & Chen, 2021].
MATERIALS AND METHODS
Reagents and reference compounds
Acetone and formic acid purchased from Avantor Performance Materials Poland S.A. (Gliwice, Poland) were used for the extraction process. The solvents used were of analytical grade. Liquid chromatography-mass spectrometry (LC-MS) grade methanol and formic acid with a minimum purity of 98% were acquired from Sigma-Aldrich (St. Louis, MO, United States). Deionized water, obtained through a Milli-Q purification system (Merck, Darmstadt, Germany), was utilized in the experiments.
Plant material and extraction process
Plant material from five different cultivars of B. vulgaris (Snow Ball, Boldor, Cylindra, Rhubarb, and Round Dark Red) and five varieties of G. mihanovichii grafted cacti (orange, green, yellow, red, and pink) was used in the study, as illustrated in Figure 1.
Figure 1
Beta vulgaris of selected cultivars - Boldor and Snow Ball (A) and Gymnocalycium mihanovichii grafted cacti in colors: yellow, green, orange, pink, and red (B).

The beet seeds were purchased from the company W. Legutko, located in Jutrosin, Poland. B. vulgaris plants were cultivated in the botanical garden of the University of Agriculture in Cracow, Poland, from June to September 2022 by a unit specializing in plant cultivation. Orange, green, and yellow cacti were imported from a Dutch plantation, whereas the red and pink varieties originated from a Polish company named Tomaszewski in Warsaw. After harvesting, the leaves of beets, as well as the upper colored parts of the cacti, were washed, weighed, and directly subjected to the extraction process.
The leaves of beets as well as the upper peeled parts of cacti were individually blended in a household blender. Appropriate extraction procedures were employed to extract betaxanthins from 100 g of beets and cacti. The beets were extracted using the maceration method [Celli & Brooks, 2017], utilizing 200 mL of a 50% (v/v) aqueous acetone solution. In contrast, the cacti underwent extraction using 300 mL of a 50% (v/v) aqueous acetone solution enriched with 1% formic acid. The entire extraction process lasted for 90 min, maintaining ambient temperature and ensuring darkness. Upon completing the extraction, the obtained extracts were filtered under reduced pressure to eliminate potential impurities, then evaporated using a vacuum evaporator (Hei-VAP Advantage, Heidolph, Germany) at 25°C, and lyophilized in a freeze dryer (Christ, Osterode am Harz, Germany). After lyophilization, the resulting extracts were weighed. The obtained crude extracts, following appropriate preparation, were employed for analysis using UV-Vis spectroscopy and LC-MS techniques.
Spectrophotometric quantification of total betaxanthins
A quantitative analysis of the total betaxanthin content was conducted using spectrophotometry with the Tecan Infinite 200 microplate reader (Grödig/Salzburg, Austria) [Stintzing et al., 2003]. Triple measurements of absorption were performed for the extracts from both beets and cacti, dissolved in water, with each sample having a volume of 200 μL. Spectrophotometric measurements were carried out in the range of 350 to 750 nm with a 1 nm step at a temperature of 25°C. Total betaxanthin content (BC) was calculated for absorbance measured at 474 nm (λmax) according to Equation (1):
where: A is the absorbance, DF is the dilution factor, l is the path length (0.53 cm) of the microplate, ε is the molar absorption coefficient for betaxanthin (4.80×104 cm−1M−1), and MW is molecular weight (339 g/mol) [Stintzing et al., 2002]. Results were expressed in mg of pigment per 100 g of dry extract (DE) and in mg of betaxanthins per kg of fresh weight (FW) of plant material.HPLC-DAD-ESI-MS analysis of betaxanthins
Before analysis, all samples were carefully diluted in demineralized water (15 mg per 500 μL of water) and briefly centrifuged at 3,000×g for 5 min using a centrifuge (Hermle Z323K, Gosheim, Germany). The betaxanthin profiles in beets and cacti were determined using the HPLC-DAD-ESI-MS technique (LCMS-8030 system, Shimadzu, Kyoto, Japan). The LC-MS system included a precise SIL-20ACXR autosampler, an efficient degasser, and a binary pump LC-20ADXR Nexera. Samples were separated using a Kinetex C18 chromatographic column (Phenomenex, Torrance, CA, United States) with dimensions of 100 mm length × 4.6 mm i.d., containing 5.0 μm particles and protected by a 4 mm length × 2 mm i.d. guard column of the same material (Phenomenex). The column temperature was maintained constant at 40ºC. Analyses were carried out using a two-component gradient. The mobile phase consisted of methanol (A) and 2% formic acid in water (B). The flow rate was 0.5 mL/min, and 10 μL of the sample was injected for analysis. The solvent gradient system for extracts was as follows: 1% A in B at 0 min, gradient to 11% A in B at 12.0 min, 60% A in B at 24 min, and then gradient to 90% A in B at 24.01 min. UV/Vis spectra were collected using a DAD detector model SPD-M20A (Shimadzu).
In ESI-MS analyses conducted in the positive electrospray ionization mode, the capillary voltage was set at 4.5 kV, and the capillary temperature was maintained at 250°C. ESI-MS data were recorded in the scan mode with m/z ranging from 100 to 2,000 Da and the selected ion monitoring (SIM). LabSolution software version 5.91 SP1 (Shimadzu) was used for data acquisition in the HPLC-DAD-ESI-MS configuration.
Reference standards from B. vulgaris cv. Chrobry [SpórnaKucab et al., 2023] and P. grandiflora Hook. extracts [Spórna-Kucab et al., 2022], which contained previously identified betaxanthins, were used to identify individual betaxanthins in the extracts.
The quantitative analysis of individual betaxanthins was carried out by determining peak areas from MS chromatograms of B. vulgaris and G. mihanovichii extracts. The total betaxanthin content in the examined extracts was previously determined through spectrophotometric method. All samples were analyzed in three independent LC-MS runs.
Statistical analysis
The data were presented as mean and standard deviation (SD) based on three independent analyses. Statistical analysis was conducted using Statistica software version 7.1 (StatSoft, TIBCO Software Inc., Palo Alto, CA, United States), employing one-way analysis of variance (ANOVA) and the Tukey post hoc test, with a significance level of α set at 0.05. p-Values below 0.05 were considered statistically significant. The statistical analysis was performed separately for B. vulgaris leaves and G. mihanovichii grafted cacti.
RESULTS AND DISCUSSION
Betaxanthins in G. mihanovichii grafted cacti
Studies on betalain profiles in their numerous sources demonstrate that betacyanins were often found in conjunction with betaxanthins [Cai et al., 2005a; Otálora et al., 2020; Spórna-Kucab et al., 2013, 2018, 2022, 2023; Wybraniec et al., 2010; Xie & Chen, 2021]. One of the sources of betacyanins are cacti. Previously, a total of 32 different betacyanins were identified in the red variety of Gymnocalycium mihanovichii cv. Hibotan scions [Wybraniec et al., 2010]. However, there is a lack of information regarding the presence of individual betaxanthins. Interestingly, according to a previous source [Wybraniec et al., 2010], the yellow-orange color is not attributed to the presence of betaxanthins but rather to the synthesis of carotenoids in cacti. In the mentioned study, no betaxanthins were detected in any of the analyzed violet, pink, and red cacti. Here, based on chromatographic, spectrophotometric, and mass-spectrometric data (Table 1), the presence of four polar betaxanthins was indicated in yellow, orange, red and pink varieties of G. mihanovichii grafted cacti: histidine-Bx (1), histamine-Bx (2), serine-Bx (3), and proline-Bx (11). The chemical structures of compounds 1 (predominant), 2 and 11 are shown in Figure 2. All the betaxanthins were solely identified in the red cactus variety, while they were absent in the green variety. Notably, the yellow and orange varieties lacked histamine-Bx (2), while the pink variety contained only histidine-Bx (1) and proline-Bx (11) (Figure 3). The samples displayed a range of total betaxanthin contents, varying from 0.09 to 1.55 mg/kg FW. The highest levels were determined in the red cactus (1.55 mg/kg FW) followed by the pink variety (1.29 mg/kg FW). Subsequently, the orange and yellow varieties had lower contents (0.22 and 0.09 mg/ kg FW, respectively) (Table 2).
Figure 2
Chemical structures of predominant betaxanthins detected in the leaves of Beta vulgaris (compounds 2, 4, 8, 9, 10, 11, 14, and 15) and Gymnocalycium. mihanovichii grafted cacti (compound 1).
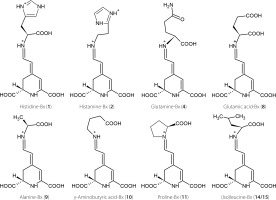
Table 1
Chromatographic, spectrophotometric, and mass-spectrometric data of the analyzed betaxanthins of leaf extracts of white, yellow and red Beta vulgaris cv. Snow Ball, Boldor, Cylinder, Rhubarb, and Round Dark Red, as well as Gymnocalycium mihanovichii grafted cactus (orange, green, yellow, red, and pink) extracts.
Table 2
The content of total betaxanthins and individual betaxanthins in extracts from the leaves of five cultivars of Beta vulgaris and five varieties of Gymnocalycium mihanovichii grafted cactus (mg/100 g dry extract, DE) and the total betaxantin content in fresh weight (FW) of beet leaves and grafted cacti, respectively, (mg/kg FW), determined by HPLC-DAD-ESI-MS.
[i] Data are expressed as mean ± standard deviation (n=3). The superscript letters within each row (a–e or A–D) mean significant differences between results (p<0.05). The statistical analysis was conducted separately for beet leaves and grafted cacti. The assigned betaxanthin numbers correspond to those listed in Table 1. –, Not detected.
The total betaxanthin content determined in G. mihanovichii grafted cacti was lower than that of the yellow pulp of the Opuntia ficus-indica fruits, which was 275 mg/kg FW [Fernández-López et al., 2018]. Some similarities with G. mihanovichii grafted cacti were observed regarding the betaxanthin profile of the yellow O. ficus-indica fruits [Fernández-López et al., 2018]. Importantly, two specific compounds 1 and 11 were found in both profiles. However, in the case of O. ficus-indica fruits, compound 11 prevailed as the dominant pigment. In turn, compound 1 prevailed in G. mihanovichii grafted cacti scions, particularly in the orange, red, and pink varieties (Table 2). Additional betaxanthins, namely glutamine-Bx (4) and 5-methionine-betaxanthin, were detected in the yellow O. ficus-indica [Fernández-López et al., 2018], in contrast to their absence in G. mihanovichii grafted cacti. In turn, Kugler et al. [2007] analyzed the betaxanthin profile of O. ficusindica cv. Gialla and identified a total of 13 distinct betaxanthins. Proline-Bx (11) was reported as the major betaxanthin. However, in this complex profile, histamine-Bx (2) was absent, in contrast to betaxanthin profile of the red variety of G. mihanovichii grafted cacti analyzed in our study (Figure 3, Table 2). Similarly, betaxanthin 2 and two other compounds, histidine-Bx (1) and serineBx (3), were not identified in the betaxanthin profile of O. dillenii [Betancourt et al., 2017].
Figure 3
Chromatogram monitoring selected ions (ESI-MS) in the positive ion mode for betaxanthins from Gymnocalycium mihanovichii grafted cacti varieties: (A) orange, (B) yellow, (C) red, and (D) pink. The numbers and names are available in Table 1.

The highest total betaxanthin content of G. mihanovichii grafted cacti found in the red variety (Table 2) was consistent with a previous study of 35 different cactus varieties, which showed that red varieties generally had the highest content of betaxanthins [Pérez-Loredo et al., 2016]. Interestingly, similar results were obtained in the case of beetroots, where red cultivars contained higher amounts of betaxanthins compared to the white and yellow varieties [Spórna-Kucab et al., 2023]. The total betaxanthin content in the red G. mihanovichii grafted cacti of 1.55 mg/kg FW (Table 2) was higher than that determined in green fruits of O. ficus-indica originating from the United States and reaching 1.7 mg/kg DW [Pérez-Loredo et al., 2016]. Here, analyses of the green variety of G. mihanovichii grafted cacti failed to identify any betaxanthins (Table 2).
The highest total content of betaxanthins in the G. mihanovichii grafted cacti extract was determined in the red variety, amounting to 19.5 mg/100 g DE, followed by the pink variety at 13.9 mg/100 g DE, then the orange variety at 5.3 mg/100 g DE, and finally the yellow variety at 1.4 mg/100 g DE. The compound profile of G. mihanovichii grafted cacti is not complex. Therefore, the isolation of histidine-Bx (1) from the extract of red and pink G. mihanovichii grafted cacti is indeed achievable.
Betaxanthins in B. vulgaris leaves
For the first time, the identification of betaxanthins has been accomplished in the leaves of B. vulgaris cv. Snow Ball, Boldor, Cylindra, Rhubarb Chard, and Round Dark Red. Analysis conducted by utilizing the HPLC-DAD-ESI-MS technique enabled a tentative identification of 15 betaxanthins: histamine-Bx (2), serine-Bx (3), glutamine-Bx (4), ornithine-Bx (5), ethanolamine-Bx (6), lysine-Bx (7), glutamic acid-Bx (8), alanine-Bx (9), γ-aminobutyric acid-Bx (10), proline-Bx (11), valine-Bx (12), 3-methoxytyramine-Bx (13), isoleucine-Bx (14), leucine-Bx (15), and tryptophan-Bx (16) (Figure 4, Table 1). The chemical structures of the dominant betaxanthins in beet leaves are shown in Figure 2. The presence of all compounds, except for histidine-Bx (1), was confirmed based on the root extract of B. vulgaris cv. Chrobry obtained in our previous study [Spórna-Kucab et al., 2023]. In turn, the extract from P. grandiflora analyzed previously [Spórna-Kucab et al., 2022] played a crucial role in betaxanthin 1 identification.
The qualitative profile of betaxanthins varied depending on the beet cultivar. Fifteen betaxanthins were identified only in the Rhubarb cv. (Figure 4). In the other cultivars, various combinations of betaxanthins were noticeable. Remarkably, the Snow Ball cv. had the smallest diversity of betaxanthins, including 6 compounds.
Figure 4
Chromatogram monitoring selected ions (ESI-MS) in the positive ion mode for betaxanthins from leaves of Beta vulgaris cultivars: (A) Snow Ball, (B) Boldor, (C) Cylindra, (D) Rhubarb and (E) Round Dark Red. The numbers and names are available in Table 1.

Research on chemical compound profiles in different parts of a plant contributes to a more comprehensive understanding of the influence of chemical composition on plant properties and the selection of appropriate sources of specific natural compounds. The B. vulgaris cv. Boldor and Cylindra have already been analyzed for their betaxanthin profiles in their root systems in our previous study [Spórna-Kucab et al., 2023], which revealed a complex profile, including 23 betaxanthins. Additionally to most compounds indentified in B. vulgaris leaves in the current study, the presence of histidine-Bx (1), asparagine-Bx, arginine-Bx, glycine-Bx, threonine-Bx, dopa-Bx, dopamine-Bx, tyrosineBx, methionine-Bx, and phenylalanine-Bx was detected. It is worth noting that histamine-Bx (2) was not detected in the root samples. In contrast, it was present in the leaves of all B. vulgaris cultivars (Figure 4, Table 2). This observation is in agreement with previous research on the roots of red and yellow beet cv. Burpees Golden, as well as the leaves of Swiss chard cv. Bright Lights, which unequivocally established the presence of histamine-Bx (2) solely in the leaves, and its absence in the beet roots [Kugler et al., 2004, 2007].
Research on Swiss chard revealed the betaxanthin profile in leaves, emphasizing their potential as an equally rich source of betaxanthins [Kugler et al., 2004, 2007]. The presence of a total of 25 betaxanthins was established, including compounds that were not identified in the current study in the leaves of B. vulgaris. Specifically, these compounds were histidineBx (1), asparagine-Bx, aspartic acid-Bx, glycine-Bx, threonine-Bx, dopa-Bx, tyrosine-Bx, dopamine-Bx, methionine-Bx, tyramine-Bx, and phenylalanine-Bx. Whereas most of betaxanthins have previously been documented in Swiss chard [Kugler et al., 2004, 2007], ornithine-Bx (5) has been reported in the current study for the first time in leaves of all B. vulgaris cultivars. Interestingly, its presence was recently confirmed in roots of B. vulgaris Forono, Tytus, Ceryl, Boldor, and Chrobry cultivars [Spórna-Kucab et al., 2023].
The total betaxanthin content was the highest in fresh leaves of the yellow beet variety cv. Boldor (20.4 mg/kg FW), followed by the red cultivars: Cylindra, Rhubarb, and Round Dark Red (18.8 15.4, and 13.4 mg/kg FW, respectively), with the lowest value determined in the white cultivar Snow Ball (3.43 mg/kg FW). This study revealed lower total betaxanthin content in the leaves, ranging from 3.34 to 20.4 mg/kg FW, compared to 107 mg/kg FW assayed in the case of Swiss chard [Gamba et al., 2021; Kugler et al., 2007]. Here, the red beet cv. Cylindra had a similar betaxanthin content of 18.8 mg/kg FW compared to the yellow cv. Boldor which had 20.4 mg/kg FW. This may emphasize the underestimated significance of betaxanthins in red beets.
Dried extracts obtained from the leaves of the yellow beet cv. Boldor had the highest total betaxanthin content, reaching 161.2 mg/100 g DE. In contrast, the white cv. Snow Ball accumulated the lowest betaxanthin quantities, 48.0 mg/100 g DE.
Red cultivars, on the other hand, had contents between these values (158.5, 112.5, and 85.5 mg/100 g DE for cultivars Cylindra, Round Dark Red, and Rhubarb, respectively). Betaxanthin content in dried extracts from the B. vulgaris roots of five cultivars (four red: Ceryl, Chrobry, Forono, and Tytus, as well as one yellow: Boldor) has been previously studied [Spórna-Kucab et al., 2023]. The results of that study revealed notably elevated betaxanthin content, with the highest levels noted in red beet cultivars: Ceryl, Chrobry, Forono, and Tytus (from 669 to 1231 mg/100 g DE for peel and 528 to 609 mg/100 g DE for flesh), rather than in the yellow ones (317 and 574 mg/100 g DE for flesh and peel, respectively). These findings suggest that, in contrary to the common perception that yellow beets are a rich source of betaxanthins, their value may be surpassed by those found in red cultivars. Within the leaves of B. vulgaris, substantial levels of betaxanthins were likewise detected in the red cultivars (Table 2).
Different pigments were found to predominate in various beet cultivars. In leaf extracts of all examined cultivars of B. vulgaris, γ-aminobutyric acid-Bx (10) was predominant, except for cv. Snow Ball. In the extract of cv. Boldor, there were notable levels of γ-aminobutyric acid-Bx (10) at 42.7 mg/100 g DE, glutamine-Bx (4) at 25.7 mg/100 g DE, and glutamic acid-Bx (8) at 24.9 mg/100 g DE. In the extract of cv. Rhubarb, the dominance of three pigments was noted: γ-aminobutyric acid-Bx (10) at 26.4 mg/100 g DE, alanine-Bx (9) at a level of 15.9 mg/100 g DE and proline-Bx (11) at a level of 14.6 mg/100 g DE. Compound 10 also dominated in the leaf extract of cv. Round Dark Red, with a quantity of 44.8 mg/100 g DE and in cv. Cylindra, with a quantity of 32.2 mg/100 g DE. Isoleucine-Bx (14) and histamine-Bx (2) were the major pigments in the extract of cv. Snow Ball, with contents of 15.4 mg/100 g DE and 14.7 mg/100 g DE, respectively. In leaf extract of cv. Cylindra, γ-aminobutyric acid-Bx (10) clearly dominated, reaching a level of 32.2 mg/100 g DE. In this cultivar, the following compounds stand out as well: isoleucine-Bx (14) with a content of 19.6 mg/100 g DE, histamine-Bx (2) with a content of 19.5 mg/100 g DE, glutamic acid-Bx (8) with a content of 18.0 mg/100 g DE, alanine-Bx (9) and leucine-Bx (15) with contents of 17.5 mg/100 g DE and 17.6 mg/100 g DE, respectively. These betaxanthins contribute unique properties to each cultivar, arousing curiosity about their potential health benefits.
Glutamine-Bx (4) and γ-aminobutyric acid-Bx (10) were predominant betaxanthins in B. vulgaris cv. Boldor, both in the roots [Spórna-Kucab et al., 2023] and in the analyzed leaf extracts. Moreover, betaxanthin 4 prevailed in all studied root extracts (Snow Ball, Boldor, Cylinder, Rhubarb, and Round Dark Red), except for the peel of yellow B. vulgaris (cv. Boldor) where betaxanthin 11 predominated. This underscores the significance of these compounds in the overall composition of beetroot, encompassing both its roots and leaves.
CONCLUSIONS
In conclusion, this study has provided valuable insights into the presence and distribution of betaxanthins in different varieties of Gymnocalycium mihanovichii grafted cacti and the leaves of Beta vulgaris cultivars. The research revealed distinct betaxanthin profiles in different varieties of these plants, shedding light on their potential as sources of these pigments. In G. mihanovichii grafted cacti, preliminary investigations indicated the presence of four polar betaxanthins, i.e., histidine-Bx (1), histamine-Bx (2), serine-Bx (3), and proline-Bx (11). The total betaxanthin contents varied among these varieties with the highest levels observed in the red one, followed by the pink, orange, and yellow ones. Betaxanthins were not identified in the green cactus variety. When comparing G. mihanovichii grafted cacti to other studied cacti, it becomes evident that while the betaxanthin content in G. mihanovichii grafted cacti was lower, its profile was unparalleled in other plants.
Betaxanthins in the leaves of various of B. vulgaris cultivars, including Snow Ball, Boldor, Cylindra, Rhubarb Chard, and Round Dark Red, were identified for the first time ever. The qualitative profile differed among cultivars, with the greatest diversity found in the Rhubarb cultivar. Their contents in B. vulgaris leaves varied among cultivars as well, with yellow cultivar Boldor exhibiting the highest total betaxanthin content. It is worth noting that the contents found in the red cultivars were also very high, confirming that the leaves of these beet cultivars are also an excellent source of betaxanthins.
In summary, this research has expanded our knowledge of betaxanthins in G. mihanovichii grafted cacti and leaves of B. vulgaris, highlighting them as sources with diverse pigment profiles.

