INTRODUCTION
Betacyanins (a subgroup of betalain plant pigments) are imine derivatives of betalamic acid which forms betanidin by condensation with (2S)-5,6-dihydroxy-2,3-dihydro-1H-indole-2-carboxylic acid (cyclo-DOPA). The above condensation results in the formation of the basic structural unit of betacyanins [Strack et al., 2003; Tanaka et al., 2008]. Other betacyanins, namely betacyanin glycosides as well as their acylated derivatives, can be regarded formally as formed upon the condensation of betalamic acid with glycosylated (as well as subsequently acylated) derivatives of cyclo-DOPA, however, the order of condensation, glycosylation and acylation is still a subject of debate [Miguel, 2018; Slimen et al., 2017]. A characteristic attribute of all betacyanin structures is the presence of nitrogen atoms derived from the dihydroindole moiety and betalamic acid [Mabry & Dreiding, 1968]. As indicated by recent literature reports, betacyanins can currently be divided into 7 subtypes based on the following primary structures: betanin, melocactin, amaranthin, oleracin, apiocactin, gomphrenin and glabranin, which differ in substituents [Kumorkiewicz-Jamro et al., 2021].
The most known betacyanin is betanin (betanidin 5-O-β-glucoside). It has a glucosyl moiety attached to the carbon atom at the position C-5 [Gandía-Herrero et al., 2010] and is sourced mainly from Beta vulgaris L. (beetroot, red beet). Betanin is a compound with the most thoroughly studied physicochemical, antioxidant and bioactivity properties [Amjadi et al., 2018; da Silva et al., 2019; Esatbeyoglu et al., 2015; Knorr et al., 2015; Kumorkiewicz-Jamro et al., 2020; Wendel et al., 2016; Wybraniec et al., 2013; Wybraniec & Michalowski, 2011]. Compounds from the gomphrenin group can be found mainly in plants of the Basellaceae family, particularly in Basella alba L. and its variety Basella alba L. var. “Rubra” (Malabar spinach) [Arokoyo et al., 2018; Cai et al., 2003; Khan & Giridhar, 2015].
B. alba is native to the Indian subcontinent, Southeast Asia and New Guinea where it is widely used as a leaf vegetable [Adhikari et al., 2012]. It is a fast-growing, soft-stemmed vine, reaching 10 m in length with thick, semi-succulent, heart-shaped leaves. It has small, dark blue stone fruits and distinctive branched climbing stems [Chaurasiya et al., 2021].
The total betacyanin content in the mature fruits of B. alba plants is 42.0 mg/100 g and in those of B. alba “Rubra” is 86.6 mg/100 g. Gomphrenin is the major compound in these plants – ca. 40% of all betacyanins in both varieties, whereas other compounds found are derivatives the properties of which are still not fully explored [Sutor-Świeży et al., 2022a].
The stability of betalains in solutions is curbed by environmental factors such as: pH, water activity, light, presence of oxygen, temperature, presence of enzymes, compounds with antioxidant activity or metal cations [Herbach et al., 2006; Stintzing & Carle, 2004]. Betalains are stable in the pH range from 3 to 7, with environment pH 4–6 found optimal for their stability, which has been reported to increase in anaerobic conditions [Azeredo, 2009]. In the optimal pH range, temperature is a key determinant of their degradation [Kapadia et al., 2003].
However, by appropriately selecting the reaction conditions, it is possible to generate decarboxylated derivatives with potentially different antioxidant and bioactive properties [Kumorkiewicz et al., 2020; Wybraniec & Mizrahi, 2005]. The use of stabilizing agents, such as ethylenediaminetetraacetic acid (EDTA) or citrates, enables even more precise control of the resulting thermal decarboxylation products [Herbach et al., 2006; Pasch & von Elbe, 1979; Sutor-Świeży et al., 2022b]. Recently, we have conducted controlled thermal modifications of gomphrenin structure in order to obtain decarboxylated derivatives, and then, isolated the purified compounds [Sutor-Świeży et al., 2024].
Due to the potential health-promoting properties of the betacyanin compounds as well as still insufficient study on this topic, the important approach of the present study was also to find out if the obtained gomphrenin derivatives express health-promoting activities. Growing body of literature report anti-cancer [Kumar et al., 2015a, b] and inflammatory [Lin et al., 2010] potential of gomphrenin. However, the studies conducted so far have mainly focused on extracts, but not isolated compounds, while the activity of decarboxylated gomphrenin derivatives has not been studied yet. Therefore, in this contribution, we aimed at verifying if decarboxylated and acylated gomphrenin derivatives may affect cell proliferation and aggravate cancer cell survival due to programmed cell death.
To achieve this goal, two Duke’s type C colorectal adenocarcinoma cell lines were employed. The HT-29 cell line was obtained from a primary (“in situ”) colon tumor, and SW620 cancer cells was derived from a metastatic site. Non-cancerous CHO-K1 cell line was used for comparative purposes.
MATERIAL AND METHODS
Reagents
Formic acid, acetone, methanol (mass spectrometric grade), and water were obtained from Sigma Chemical Co. (St. Louis, MO, USA).
Plant material
The plants of B. alba were cultivated in a greenhouse of the University of Agriculture (Faculty of Biotechnology and Horticulture) in Cracow, Poland, according to the recently presented procedure [Sutor-Świeży et al., 2024]. The plants were grown at controlled temperature and moisture for proper flowering and fruiting (photo of the plants is shown in Figure S1 in Supplementary Materials). Matured B. alba fruits were collected and used for analyses.
Preparation of B. alba fruit extract
B. alba fruit extract was prepared according to a recently published procedure [Sutor-Świeży et al., 2024]. Typically, 1 kg of the fruits was extracted 3 times with water (1 L) for 30 min at room temperature, and the combined extracts were centrifuged as well as filtered through a 0.2 mm i.d. pore size filter and a bed of 0.063/0.200 mm silica (J.T. Baker, Deventer, Holland). Purification of 0.03 M HCl acidified extract was performed by means of open column chromatography (150 mm height × 50 mm i.d.) on strongly acidic cation exchange resin (Strata X-C, Phenomenex, Torrance, CA, USA) acidified with 0.03 M HCl before adsorption of the pigments. The betacyanin fraction was eluted with water after prior purification with 1% formic acid, and the eluates were pooled before concentration using a rotary evaporator under reduced pressure at 25°C. The extracts were stored at -20°C for preservation over several weeks before exact experiments.
Generation of decarboxylated gomphrenins in semi-preparative scale for bioactivity assays
Decarboxylated gomphrenins at specific positions were obtained according to recently published procedures [Sutor-Świeży et al., 2024] in a diluted 2-L purified fruit extract solution (total betacyanin concentration of 30–60 μM). Pigment 17-decarboxy-gomphrenin was generated in a fruit extract aqueous solution (60 μM total betacyanins) containing 100 mM citric acid, following 3-h heating at 65°C. Similarly, the pigments 2-decarboxy- and 2,17-bidecarboxy-gomphrenin were generated in a fruit extract aqueous solution (30 μM total betacyanins) with 100 mM citric acid, subjected to 2–3 h of heating at 70°C. The resulting solutions were adsorbed onto a Chromabond silica C18 (Macherey & Nagel Co. Düren, Germany) column, and the pigments were subsequently eluted with a formic acid/acetone/water (1/50/49, v/v/v) solution and concentrated before preparative high-performance liquid chromatographic (HPLC) separation.
Isolation and purification of gomphrenin pigment from extracts
Gomphrenin and acylated gomphrenins were separated from B. alba purified fruit extract, and decarboxylated gomphrenins were isolated from the reaction mixtures obtained from the purified extract by heating (described in the previous section) on a HPLC semipreparative column Synergy Hydro-RP 250 mm × 30 mm i.d., 10 μm (Phenomenex), along with a 20 mm × 25 mm i.d. guard column of the same material (Phenomenex), according to a previously described procedure [Sutor-Świeży et al., 2024]. A typical gradient system consisting of 1% aqueous formic acid (solvent A) and acetone (solvent B) was used as follows: 0 min, 14% B; increasing to 10 min, 20% B; increasing to 15 min, 24% B; increasing to 28 min, 28% B; increasing to 36 min; 75% B. The injection volume was 25 mL, and a flow rate was 45 mL/min. Detection was performed using a UV-Vis detector at two selected wavelengths from the range of 510–540 nm, depending on the separated pigments, at column temperature of 22°C. The fraction obtained after concentration in a rotary evaporator at 25°C under reduced pressure were stored at −20°C for further analyses.
Chromatographic analysis with detection by low- and high-resolution mass spectrometric systems
A low-resolution mass spectrometric system LCMS-8030 (Shimadzu, Kyoto, Japan) controlled by LabSolutions software (v. 5.60 SP1, Shimadzu) equipped with HPLC pumps (LC-20ADXR), a SIL-20ACXR injector and an SPD-M20A detector was applied for the identification and determination of the obtained analytes. The same conditions as recently reported [Sutor-Świeży et al., 2024] were applied for the analytical separation of the pigments with the eluent gradient composed of aqueous 2% formic acid (solvent A) and methanol (solvent B): (t (min), % A), (0, 90), (12, 60), (15, 20), (19, 20). Detected pigments were readily assigned according to their chromatographic, optical and mass spectro-metric properties presented in Table 1 based on our previous study [Sutor-Świeży et al., 2024].
Table 1
Chromatographic, spectrophotometric and low-resolution mass spectrometric data of the gomphrenin-based pigments obtained from Basella alba L. fruits.
The LC-MS analyses with high-resolution mass spectrometric detection were performed using an Orbitrap Exploris™ 240 Mass Spectrometer (Thermo Fisher Scientific, Brema, Germany) according to the conditions provided in our recent report [Sutor-Świeży et al., 2024]. The detector was coupled to an HPLC Dionex Ulti-Mate 3000 chromatographic separation system operating under Xcalibur software (Thermo Fisher Scientific) version 4.5.445.18.
Cell cultures
The following certified cell lines were derived from the American Type Cell Culture (ATCC) collection (LGC Standards-ATCC, Teddington, Great Britain): HT-29, Duke’s type C colorectal adenocarcinoma (HTB-23); SW 620, Duke’s type C colorectal adenocarcinoma (CCL-227), and CHO-K1 normal ovary cells (CCL-61). The HT-29 and SW 620 cells were grown in Dulbecco’s Modified Eagle’s Medium, DMEM (Gibco Laboratories, Grand Island, NY, USA), and the CHO-K1 cells were cultured in DMEM/F12 medium (Gibco Laboratories). All the media were supplemented with 10% (v/v) fetal bovine serum (FBS, Eurx, Gdańsk, Poland) and with an antibiotic solution (100 IU/mL penicillin, 0.1 mg/mL streptomycin; Sigma-Aldrich, Seelze, Germany). Prior to experiments, the cells were cultured for 24 h to obtain monolayers and were kept under standard culture conditions (humidified atmosphere, 37°C, 5% CO2 in air), as described elsewhere [Adach et al., 2016; Tyszka-Czochara et al., 2021]. The tested compounds (listed in Table 1) were dissolved in an appropriate medium before cell culture experiments. Each experiment was repeated three times.
Cytotoxicity assays
For experiments, 100 μL of a suspension of exponentially dividing HT-29 or SW 620 cells at density of 1.0×105 cells/mL (CHO-K1 at density of 1.5×105 cells/mL) were inserted onto 96-well cell culture plates (BD Biosciences, San Jose, CA, USA) and incubated for 24 h. The 3-[4,5-dimethylthiazol-2yl]-2,5-diphenyl tetrazolium bromide (MTT) colorimetric assay was performed for the detection of cell proliferation, as described previously [Adach et al., 2015]. The cells were exposed to the tested compounds (listed in Table 1), each at concentrations of 0.01, 0.1, 1 and 10 mg/mL, for 24 h. The cells cultured in the medium with no additives served as positive controls (100% of growth). The cytotoxic effect of anti-cancer drug, Doxorubicin (DOX), at a concentration of 10 μg/mL was also determined.
The medium was removed after incubation, and the MTT reagent (Sigma-Aldrich) was added to each well, and incubation was continued for 1 h. During incubation, generated MTT formazan was dissolved in dimethyl sulfoxide (DMSO, Sigma-Aldrich), and the absorbance was recorded at 570 nm (with reference λ at 630 nm) in a microplate reader (Infinite M200 Pro, Tecan, Grödig, Austria). The amount of reduced MTT salt was proportional to the number of metabolically active (living) cells in the population exposed to gomphrenins. The results were expressed as cell proliferation (% of control cells).
Apoptosis and necrosis detection
HT-29 and SW 620 cells were seeded at density of 1.0×105 cells/mL (CHO-K1 at density of 1.5×105 cells/mL) into 6-well cell culture plates (Sarstedt AG&Co. KG, Nümbrecht, Germany) and incubated for 24 h to establish cell monolayer. Then, they were treated for 24 h with the tested pigments at concentrations of 0.01, 0.1, 1 and 10 mg/mL. Control cells were exposed for 24 h to the medium only. DOX at a concentration of 10 μg/mL was used in experiments as cytotoxicity control. For cell death detection, the cells were detached with a trypsin/EDTA solution (Gibco Laboratories), washed with Dulbecco’s Phosphate Buffered Saline (DPBS, Gibco Laboratories) and centrifuged at 350×g for 5 min. Then, cell suspensions were exposed for 30 min in the dark to appropriate fluorescent dyes in a binding solution according to the recommended protocol (Biotium, Inc., Fremont, CA, USA). Fluorescent dyes used in the measurement were 488-AnnexinV (Biotium, excitation maximum at 490 nm/emission maximum at 515 nm), Ethidium homodimer (Biotium, EthD-III; excitation maximum at 528 nm/emission maximum at 617 nm) and SYTO 41 Blue Fluorescent Nucleic Acid Stain (Thermo Fisher Scientific Inc., Waltham, MA, USA, excitation maximum at 483 nm/emission maximum at 503 nm, used for proper discrimination between cells and debris). The assessment of apoptotic and necrotic cells was performed with a flow cytometer (FACSCanto10C) operated under software BD FACSCanto (BD Biosciences). The cells were gated according to forward (FSC), side scatter (SSC) and appropriate fluorescence parameters as described elsewhere [Tyszka-Czochara et al., 2017]. The results were given as the percentage of apoptotic/necrotic cells of total counted cells.
Statistical analysis
All experiments were conducted in triplicate. For the biological studies, data were analyzed using a one-way analysis of variance (ANOVA) followed by Tukey’s test, and differences were considered to be significant at p<0.05. All results were expressed as mean and standard deviation (SD) of the mean. Calculations were carried out using the commercially available package, Statistica PL v.10 (StatSoft, Tulsa, OK, USA).
RESULTS AND DISCUSSION
Gomphrenin pigments isolated from B. alba fruits and their decarboxylated derivatives
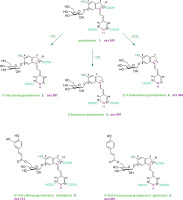
Antioxidant and anti-inflammatory properties of decarboxylated and acylated gomphrenins compared to gomphrenin, a potent bioactive betacyanin, were reported in our recent work [Sutor-Świeży et al., 2024]. In vitro screening experiments revealed also that these pigments demonstrated strong anti-inflammatory properties in lipopolysaccharide (LPS)-activated human macrophages. Therefore, in this contribution, further characterization of gomphrenin decarboxylation mechanism as well as first results on the fragmentation pathways of the novel decarboxylated gomphrenins are presented. Sufficient quantities of decarboxylated and acylated gomphrenins (Figure 1) obtained in the study enabled further bioactivity experiments. The structures of B. alba endogenous gomphrenin and acylated gomphrenins (malabarin and globosin) were analyzed previously by nuclear magnetic resonance (NMR) techniques [Sutor-Świeży et al., 2022a]. Decarboxylated gomphrenins (2-decarboxy-gomphrenin, 17-decarboxy-gomphrenin and 2,17-bidecarboxy-gomphrenin) were structurally elucidated by NMR very recently [Sutor-Świeży et al., 2024].
Figure 1
Chemical structures of novel decarboxylated gomphrenins and endogenous gomphrenin pigments obtained from Basella alba fruits.
Mechanism of gomphrenin 17-decarboxylation
In this study, 17-decarboxy-gomphrenin and 2,17-bidecarboxy-gomphrenin were generated in controlled thermal conditions in the purified B. alba extract at 65-75°C, which was elaborated previously [Sutor-Świeży et al., 2024]. Based on the previous mechanism reported for betanidin [Dunkelblum et al., 1972], the mechanism of 17-decarboxy-gomphrenin formation is presented in Figure 2. The monodecarboxylation of gomphrenin which involves the loss of the C-19 carboxyl with concomitant migration of the double bond C-17=C-18 to C-14=C-15 is proposed, which was proved previously by deuteration and NMR study on betanidin [Dunkelblum et al., 1972].
Figure 2
Formation of 17-decarboxy-gomphrenin based on the previous mechanism reported for betanidin by Dunkelblum et al. [1972].
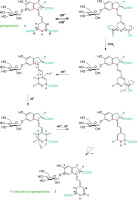
It became clear that carbons C-15 and C-17 of betanidin had interchanged to become C-17 and C-15, respectively, in decarboxybetanidin and that the decarboxylation removed C-19 of betanidin [Dunkelblum et al., 1972]. Therefore, in the current reaction scheme (Figure 2), the rotation of the dihydropyridinic moiety in the last step of the pathway resulted in establishing the final 17-decarboxylated system. The structures of the generated decarboxylated gomphrenins were confirmed by NMR analyses [Sutor-Świeży et al., 2024].
The matrix effect on the pigment generation in the extract as well as the extract purification using a cation exchanger under various conditions were decisive in obtaining the required quantities of the selected decarboxylated gomphrenins. The removal of a substantial portion of the unfavorable matrix from the extract enabled efficient protection of the pigments from degradation presumably due to the removal of unknown reactive species.
A tremendous influence on the decarboxylation pathways was observed for the addition of citrates whose high concentration played a significant role in enhancing the formation of 2-decarboxy-gomphrenin [Sutor-Świeży et al., 2024], thus highly inhibiting the Dunkelblum et al. [1972] pathway of 17-decarboxylation. The above-elaborated methods were applied for the selective generation of 2-decarboxy-gomphrenin, 17-decarboxy-gomphrenin and 2,17-bidecarboxy-gomphrenin for the following studies.
Fragmentation results on novel decarboxylated gomphrenins in the high-resolution mass spectrometric Orbitrap system
Except for confirmation of the chemical formulas [Sutor-Świeży et al., 2024], C23H27N2O11 for 17-decarboxy-gomphrenin (2) and 2-decarboxy-gomphrenin (3) as well as C22H27N2O9 for 2,17-bidecarboxy-gomphrenin (4), based on exact m/z values (Table S1 in Supplementary Materials), first detailed results on the pigment fragmentation experiments were obtained in this study by high-resolution mass spectrometric experiments in the Orbitrap system (Table S1). The list of the m/z values obtained and calculated for most abundant ions formed during the fragmentation is presented in Table S1. The elimination profiles (Figure 3) as well as chemical structures of the generated ions (Figures S2 and S3 in Supplementary Materials) are also depicted.
Figure 3
MSn fragmentation pathways of 2-decarboxy-gomphrenin, 2-dGp (A), 17-decarboxy-gomphrenin, 17-dGp (B), and 2,17-bidecarboxy-gomphrenin, 2,17-dGp (C). Mass spectrometric data are shown in Table S1 in Supplementary Materials.
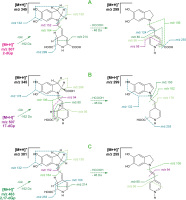
In the case of 2-decarboxy-gomphrenin (3), fragmentation experiments (Tables 1 and S1, Figure 3) of the protonated molecular ion (m/z 507) resulted in its deglucosylation (m/z 345; – Glc) and subsequent neutral loss of formic acid (m/z 299; – HCOOH), presumably from carbons C-14,15 because it leads to the dehydrogenation of C-14,15 and formation of derivatives of the pyridinium cation. The loss of formic acid can also be regarded as the concurrent elimination of H2O and CO [O’Hair et al., 2000]. Both scissions lead to the oxidation/dehydrogenation of the dihydropyridinic ring. Therefore, further fragmentation steps resulted in the formation of pyridinium-based ions (m/z 106, 80, and 56), either coupled with the elimination of the last carboxyl at C-17 (m/z 255; − CO2) or with no decarboxylation (m/z 124 and 98).
The elimination of betalamic acid and its derivatives from the starting deglucosylated precursor (3) (m/z 345) resulted mainly in the generation of ions comprising the dihydroindolic system (m/z 214, 164, and 152) as well as the indolic (m/z 150) and dehydrated indolic (m/z 132) structures.
In contrast to 3, for 17-decarboxy-gomphrenin (2), except of deglucosylation (m/z 345; − Glc), a neutral loss of formic acid (m/z 299; − HCOOH) is predicted at carbons C-2,3 instead of C-14,15, based on the previously indicated prevailing tendency of concurrent dehydrogenation and decarboxylation at carbons C-2,3 in betacyanins [Wybraniec & Michalowski, 2011] during their oxidation. These observations are supported in the current frag-mentation study by the formation of the indolic (m/z 178 and 162) instead of the dihydroindolic (m/z 214, 164, and 152) derivatives, demonstrating the dehydrogentation position in the product of HCOOH neutral loss (m/z 299) at C-2,3 (Figure 3).
Further fragmentation of the precursor ion at m/z 299 enabled detecting the indolic (m/z 150) and dehydrated indolic (m/z 132) structures, as in the case of 3. The decarboxylation of the precursor (m/z 299) at carbon C-15 enabled confirming the formation of an ion at m/z 255. In turn, the fragments detected at m/z 94 indicate the presence of a mono-decarboxylated betalamic acid derivative with the carboxyl present at carbon C-15.
Detection of pyridinium-based ions (m/z 106, 80, and 56) from both the precursors (m/z 345 and 299) confirms that their formation is feasible, as in the case of 3; however, with accompanying deformyloxylation (− HCOOH).
The fragmentation of 2,17-bidecarboxy-gomphrenin (4) resulted in the generation of a spectral pattern similar to the patterns of 2 and 3; however, with key differences. The formation of dihydroindolic (m/z 214, 164, and 152) as well as indolic (m/z 150) and dehydrated indolic (m/z 132) derivatives indicates similarities between the structure of the deglucosylated precursor 4 and deglucosylated 3. The same can be stated for the detected pyridinium-based ions (m/z 106, 94, 80, and 56) which can be generated from both the fragments of 4 (m/z 301 and 255) and were confirmed for both the deglucosylated precursors from 2 and 3. The main difference in the fragmentation patterns is the presence of an ion at m/z 100 (instead of m/z 98 detected after fragmentation of 3), indicating the presence of a carboxylated fragment generated from the mono-decarboxy-betalamyl moiety in 4 (Figure 3).
The presented in-depth interpretation of high-resolution mass spectrometric (HRMS) fragmentation coupled with NMR results obtained previously [Sutor-Świeży et al., 2024] confirmed the decarboxylated pigment structures (2-4).
Antiproliferative and proapoptotic activity of gomphrenin and its decarboxylated derivatives
Biological activity of decarboxylated and acylated gomphrenin derivatives has been poorly investigated so far. It was reported that gomphrenin-type betalains may modulate cellular function via several mechanisms in the human body [Farabegoli et al., 2017]. A potential chemopreventive effect exerted by betalains was proposed, including a systemic action in the intestine, liver and peripheral tissues [Rahimi et al., 2019]. Therefore, in the present experiments, the MTT assay was employed to determine the effect of the pigment 1-6 treatment on the survival of cancer as well as non-cancerous cells.
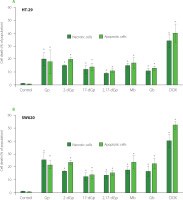
The isolated individual gomphrenin as well as decarboxylated (2-dGp, 17-dGp and 2,17-dGp) and acylated (Mb and Gb) gomphrenin derivatives (1-6) from B. alba fruits expressed toxicity against cancer cell lines, HT-29 and SW620, in particular at con-centrations 1 and 10 mg/mL. The comparative plots are presented in Figure 4. Our study demonstrated that tested betacyanins exert antiproliferative effect on both cancer cell lines at concentrations of 1 mg/mL and 10 mg/mL. The individual gomphrenin derivatives inhibited colorectal cancer cell growth to different extent. At concentration of 1 mg/mL, all the compounds (1-6) were toxic to cancer cells, but among the derivatives, 2-dGp and Mb were the most active. It should be noted that Gp, among others, at 1 mg/mL did not express any harmful effect on non-cancerous cells while it was still toxic towards tumor cells.
Figure 4
The effect of gomphrenin, Gp (A), 2-decarboxy-gomphrenin, 2-dGp (B), 17-decarboxy-gomphrenin, 17-dGp (C), 2,17-bidecarboxy-gomphrenin, 2,17-dGp (D), malabarin, Mb (E), and globosin, Gb (F) on cell proliferation as measured with MTT assay. The cells were exposed to specific concentrations of compounds for 24 h. Control cells were grown in medium only and they were treated as maximal (100%) cell viability. Doxorubicin (DOX) at a concentration of 10 μg/mL was used for comparative reasons (G). The results were expressed as the percentage of viable and metabolically active cells relative to the total counted cells. Means and standard deviations are shown (n=3). *Denotes significant difference vs. control (p<0.05).
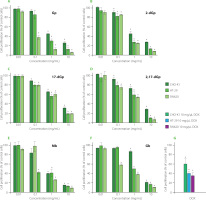
The biological action of acylated gomphrenins remains unknown; therefore, the finding that both acylated Gp derivatives can significantly restrain tumor cell growth compared to untreated cells, is of interest.
Extracts obtained from Hylocereus polyrhizus contributed to the chemopreventive effect against the formation of DNA breaks in the pUC19 plasmid under the influence of hydrogen peroxide/UV radiation and even stimulated the formation of native DNA forms [Tsai et al., 2019]. It was reported that gomphrenins had cytotoxic effects on cancer cells (SiHa) without negative effects on normal cells [Herb & Schramm, 2021; Kumar et al., 2015a]. The extract containing gomphrenin reduced the rate of T24 bladder cancer cells proliferation also without harming normal cells [Scarpa et al., 2016]. It should be emphasized that the biological action of acylated gomphrenins is not yet known, therefore, the finding that both acylated Gp derivatives can significantly restrain tumor cell growth, is of interest.
So far, only the cellular effects of some acylated polyphenol derivatives have been tested, and it was found that aromatic acyls, i.e., galloyl derivatives, appeared to improve anti-cancer efficacy of compounds through enhancement of their binding affinities to molecular targets [Essa et al., 2023]. In this report, we found that both acylated Gp (5 and 6) had great ability to hamper cell proliferation (Figure 4) and both were able to induce cell death due to apoptosis (Figure 5).
Figure 5
The effect of gomphrenin (Gp), 17-decarboxy-gomphrenin (17-dGp), 2-decarboxy-gomphrenin (2-dGp), 2,17-bidecarboxy-gomphrenin (2,17-dGp), globosin (Gb), and malabarin (Mb) on induction of cell death as measured using flow cytometry (apoptosis/necrosis assay). HT-29 cells (A) and SW620 cells (B) were exposed to 1 mg/mL concentration of compounds for 24 h. Control cells were grown in medium only and they were treated as maximal (100%) cell viability. Doxorubicin (DOX) at a concentration of 10 μg/mL was used for comparative reasons. The results were expressed as the percentage of apoptotic or necrotic cells relative to the total cells. Means and standard deviations are shown (n=3). *Denotes significant difference vs. control (p<0.05).
As reported before, betalains can contribute to the inhibition of proliferation of tumors, such as melanoma cancer cells, suppress the development of prostate and breast cancer [Khan, 2016] and delay chemically-induced lung tumorigenesis due to the progressive elimination of cancer cells via apoptosis [Zhang et al., 2013]. A programmed cell death, apoptosis, is a critical biological process that protects an organism against the development of cancer. A study performed by Tesoriere et al. [2013] on the human intestinal carcinoma cell line Caco2 showed that betalains triggered the mitochondrial-dependent apoptotic pathway, which resulted in cancer cell death.
The data presented in Figure 5, indicate that all individual acylated as well as decarboxylated gomphrenins induced cell death due to apoptosis and necrosis in human colorectal carcinoma HT-29 and SW620 cell lines. The finding that acylated and decarboxylated gomphrenins are capable of inducing cell death via apoptosis may be of interest when designing modifications of Gp derivatives with targeted biological activity. Therefore, the proapoptotic activity of gomphrenin derivatives towards cancer cells can potentially foster a very beneficial effect.
A positive control used in the study, DOX, caused massive cell death at 1 μg/mL due to both mechanisms, apoptosis and necrosis. However, the results obtained for gomphrenins should be compared to the effect of DOX with caution. Taking into account the inhibitory activity of gomphrenins, it should be noted that the reference drug showed similar effects as Gp derivatives at a concentration approximately one thousand times lower than that of the tested compounds. However, it is known that DOX effectively inhibits the proliferation of colon cancer cells by re-straining cellular divisions and that it is clinically used in cancer treatment regimens.
Both human cell lines employed in this study express morphological features of adenocarcinoma, the most common form of colon cancer which constitute up to 98% of all cases of colorectal cancer in humans. Moreover, both cell lines represent cells derived from tumor in situ at an advanced stage, which invade colon tissue (HT-29) and which pervaded the intestinal wall to the nearest lymph node (SW620) [ATCC, 2024]. Accordingly, both cell lines differ in their gene profile related to carcinogenesis and are commonly used in research models of colon cancer prevention by food compounds [van Erk et al., 2005]. In particular, all gomphrenins were toxic towards SW620 cells, which is of interest considering SW620 colorectal cancer cells characteristics, including their high proliferative capacity and chemotherapy resistance [Kawamoto et al., 2010].
We previously showed that among the tested gomphrenins, 2-dGp and Gp expressed the highest antioxidant activity in the 2,2’-azino-bis(3-ethylbenzothiazoline)-6-sulfonic acid (ABTS), ferric reducing antioxidant power (FRAP) and oxygen radical absorbance capacity (ORAC) assays, and this activity was even higher than that of a reference compound, caffeic acid [Sutor-Świeży et al., 2024]. It was shown by Zielińska-Przyjemska et al. [2012] and comprehensively reviewed by Sadowska-Bartosz & Bartosz [2021] and Bastos & Schliemann [2021] that betalains expressing high antioxidant capacity may play an important role in chemoprevention in humans. The data obtained here may be another promising prerequisite for further experiments on decarboxylated and acylated Gp derivatives’ role in the prevention of gastrointestinal tract cancer.
As reported by many authors, chronic inflammation can promote carcinogenesis at the stage of initiation and progression of cancer [Landskron et al., 2014]. Emerging data indicate that betacyanins may express regulatory action towards immune cells [Tyszka-Czochara et al., 2016]. Moreover, gomphrenins may act as potential anti-inflammatory agents via inhibition of inflammatory mediators, especially endotoxin-induced secretion of interleukin-1β (IL-1β), prostaglandin E2 (PGE2) and nitric oxide (NO) as well as the main pro-inflammatory cytokines with systemic effects [Ghonime et al., 2015], tumor necrosis factor α (TNF-α), cyclooxygenase-2 (COX-2), inducible nitric oxide synthase (iNOS) and interleukin-6 (IL-6). Our recent study demonstrated anti-inflammatory properties of several betalainic pigments, including decarboxylated and acylated gomphrenins [Sutor-Świeży et al., 2024]. It was found that 2-dGp and 17-dGp specifically targeted IL-6 production in LPS-activated human macrophages. In the previous work, we also demonstrated that acylated gomphrenins, Gb and Mb, were able to significantly reduce IL-8 secretion from human macrophages during inflammation. Since IL-8 is involved in the inflammatory process underlying the development of colon cancer [Lee et al., 2012], the ability of acylated gomphrenins to modulate inflammation via the principal regulatory cytokine, IL-8, is of interest. In line with the published results, our present study data confirm that both acylated Gp derivatives tested, Gb and Mb, are effective in restraining adenocarcinoma cells survival.
In the present experiments, gomphrenins revealed cytotoxic properties towards cancer cells at microgram and milligram concentrations. In fact, poor bioavailability of betacyanins was previously reported by a number of studies and was also attributed to gomphrenin [Khan, 2016]. Several in vivo experiments on betalain activity confirmed their limited absorption after oral intake, probably because of their degradation in the gastrointestinal tract [Khan, 2016]. At the same time, betalains as cationic compounds have high affinity for negatively-charged cell membranes, thus improving their influence on cells and their efficacy as antioxidants [Kanner et al., 2001]. It can be presumed that following the intake of a large dose of betacyanins, e.g., in the form of supplements or food enriched in particular compounds, they can still affect intestinal cells.
Considering the likely modifications of betacyanin molecules, our former experiments suggested that amaranthin-type betacyanin oxidation products were the most stable among all the tested pigments, especially during the preparative purification and isolation [Kumorkiewicz-Jamro et al., 2023]. In particular, betanin glycosylated in the C-5 position was oxidized more slowly than gomphrenin glycosylated in the C-6 position [Kumorkiewicz et al., 2018]. It is of interest whether gomphrenin oxidation products retain their intracellular action and biological activity in the body.
CONCLUSIONS
Sufficient quantities of decarboxylated gomphrenins, mostly 17-decarboxy-gomphrenin and 2,17-bidecarboxy-gomphrenin, were obtained in controlled thermal decarboxylation in the purified B. alba extract at 65–75°C, presumably according to the previously reported decarboxylation mechanism of betanidin [Dunkelblum et al., 1972]. The addition of citrates promotes the generation of 2-decarboxy-gomphrenin due to the inhibition of the Dunkelblum et al. [1972] pathway of 17-decarboxylation.
The presented in-depth interpretation of HRMS fragmentation supports the confirmation of decarboxylated pigment structures. Fragmentation pattern of 2,17-bidecarboxy-gomphrenin (4) is a combination of both the fragmentation pathways specific for 17-decarboxy-gomphrenin (2) and 2-decarboxy-gomphrenin (3). Especially, the formation of dihydroindolic as well as indolic and dehydrated indolic derivatives indicated similarities between the structures of 2-decarboxy-gomphrenin (3) and 2,17-bide-carboxy-gomphrenin (4), whereas the generation of pyridinium-based ions confirmed the presence of 17-decarboxylated and non-decarboxylated betalamic acid components specific for 2 as well as 3, respectively.
Inhibitory action of isolated gomphrenin derivatives on cancer cell lines has not been demonstrated before. We performed preliminary studies with promising findings suggesting that decarboxylated and acylated gomphrenins can exert beneficial effects and positively influence human health. All these pigments have potential for the application in the food industry.

