INTRODUCTION
Cyclocarya paliurus is a relicted tree species unique to China that survived the Ice Age and is distributed in mountainous areas at an altitude of 420–2500 meters [Zhang et al., 2021]. Due to the presence of various physiological active substances, such as flavonoids, saponins, terpenoids, polysaccharides, etc., beneficial to the human body, its leaves are often used to brew tea. Its golden color and sweet taste make it known as “sweet tea”. C paliurus leaves elicit multiple health benefits, such as preventing and treating diabetes, hypertension, hyperlipidemia, dizziness and swelling and pain, as well as reducing cholesterol level, and modulating the functions of the immune system [Chen et al., 2022b]. The leaves of C. paliurus were approved as new food raw material by the National Health and Family Planning Commission of China in 2013. However, the quality of commercially available C. paliurus tea is uneven, and the processing technology for professional production of C. paliurus tea has not yet been developed. The traditional processing of C. paliurus involves mostly direct drying, but in recent years, some producers have adopted the processing method of green tea including first its fixation followed by drying. It has not been reported in literature whether fixation is suitable for processing of C. paliurus, and how the profiles of volatile organic compounds (VOCs) and aroma compounds of C. paliurus change after fixation. Such knowledge seems important to improve the technology of processing C. paliurus leaves for tea.
Fixation is one of the critical steps in fresh leaves processing for tea. By inactivating enzymes at high temperatures, it prevents tea from turning red [Ouyang et al., 2022]. Simultaneously, a series of chemical reactions occur, such as hydrolysis and oxidation of proteins and polysaccharides, polyphenol polymerization, pigment degradation, and generation of simple compounds that are beneficial for tea quality [Wang et al., 2021a]. The main task of fixation is to form an acceptable aroma to satisfy consumers [Xu et al., 2020]. Endeavors have been made to increase the formation of “beneficial” chemicals, such as linalool, phenacetaldehyde and benzoic acid, and to avoid the presence of “unfavorable” chemicals, such as l-isoleucine, l-lysine and l-tyrosine from a tea aroma perspective [Wen et al., 2023; Xue et al., 2022].
Non-targeted metabolomics exhibits a high-sensitivity and wide-coverage, and provides a comprehensive view of metabolite changes, which has been widely utilized in tea research for the unbiased analysis and simultaneous identification of a large number of non-volatile and volatile metabolites [Ouyang et al., 2022; Qin et al., 2020]. Its advantage is that unknown metabolites can be discovered, and differences in metabolites associated with treatment states can be identified through multivariate statistical analysis. Its basic principle is to use high-throughput technology to comprehensively analyze metabolites in samples, including mass spectrometry, nuclear magnetic resonance, and other techniques. These techniques allow detecting thousands of metabolites simultaneously, resulting in comprehensive metabolome information. The analysis of metabolome data provides information on metabolite composition, metabolic pathway, and interaction between metabolites in the sample [Liu et al., 2019]. Untargeted metabolomics is a new approach with a wide range of application prospects, and has been successfully applied in investigating geo-tracing of plants [Cao et al., 2019], food processing [Chen et al., 2020], and plant bioactivity [Hasanpour et al., 2020].
Based on this, the purpose of this study was to compare the volatile compound profiles of C. paliurus tea prepared with and without fixation by untargeted metabolomics methods, to explore the impact of fixation on the aroma quality of C. paliurus tea, and to provide references for the development of processing standards for C. paliurus tea.
MATERIAL AND METHODS
Experimental material and chemicals
The C. paliurus leaves were randomly picked in Quzhou (Zhejiang, China) in May 2023.
n-Hexane (chromatographic grade) was purchased from Merck (Darmstadt, Germany), and all standards were purchased from BioBioPha Co., Ltd. (KIB, Kunming, China) and Sigma-Aldrich (St. Louis, MO, USA).
C. paliurus leaves processing
To produce tea using fixation, the fresh C. paliurus leaves (50 kg) were spread on bamboo woven round plates (Φ2 m; leaf layer thickness on each plate was 2–5 cm) for 12–24 h at 19–22°C with a relative air humidity of 55% until their water content reached about 15–20 g/100 g. Then, fixation was performed using a roller-hot air coupling fixation machine (6CST-80YJ, Shangyang Machinery Co., LTD, Zhejiang, China), with rolling temperatures of 170-180°C and fixation time of 60–120 s. After fixing, the C. paliurus leaves were dried at 80°C until the moisture content of the finished tea was less than 5 g/100 g.
Tea without the fixation step was produced by spreading out the collected leaves at room temperature as during the fixated treatment and then drying directly at 8°C until the moisture was less than 5 g/100 g.
Analysis of volatile metabolites in C. paliurus teas
Samples were weighed, immediately frozen in liquid nitrogen, and stored at −80°C until used. Before the analysis, the samples were ground to powder in liquid nitrogen. For the analysis, 500 mg of the powders were transferred immediately to a 20 mL head-space vials (Agilent, Palo Alto, CA, USA) containing NaCl saturated solution, to inhibit any enzyme reaction. The vials were sealed with crimp-top caps of tetrafluoroethylene resin (TFE)-silicone headspace septa (Agilent). The solid-phase microextraction (SPME) was carried out by oscillation of each vial at a constant temperature of 60°C for 5 min, then 120 μm VB/CWR/ PDMS extraction head (Agilent) was inserted into the sample headspace vial for 15 min of headspace extraction.
The identification and quantification of VOCs in the samples was carried out using an Agilent 7000D triple quadrupole gas chromatography–mass spectrometry (GC/MS) system (Agilent), equipped with a 30 m × 0.25 mm × 0.25 μm (5%-phenyl)-methylpolysiloxane (DB-5MS) capillary column (Agilent). Desorption of VOCs from the fiber coating was performed in the injection port of the chromatograph at 250°C for 5 min in the splitless mode. Injector temperature was maintained at 250°C. The oven temperature was set to 40°C (3.5 min), and then increased to 100°C at 10°C/min, 180°C at 7°C/min, and 280°C at 25°C/min for 5 min. The carrier gas was helium, applied at a flow rate of 1.2 mL/min. The quadrupole mass spectrometer, ion source and transmission line temperatures were set at 150°C, 230°C and 280°C, respectively. The mass spectrum was recorded in an electron shock (EI) ionization mode at 70 eV. The analytes were identified and quantified by mass spectrometry coupled with an ion-monitoring (SIM) mode.
For qualitative analysis, the Agilent MassHunter Unknowns analysis software was used to screen volatile metabolites whose similarity to the NIST11 mass spectrum library standard was >80%. By calculating the linear formula of n-alkane (C7–C40), the Kovats retention index of each compound was obtained and compared with the theoretical retention index. For quantitative analysis, the mass concentrations of the volatile metabolites were calculated with reference to the internal standard method using Equations (1) and (2) [Wang et al., 2020]:
where: Ci is the mass concentration of any compound (μg/L), Cis is the mass concentration of the internal standard (μg/L) (ethyl caprate, 20 mg/L), Ai is the chromatographic peak area of any compound, Ais is the chromatographic peak area of the internal standard, Xi is the content of compound i in the sample to be measured (μg/g), Vi is the volume of the sample i (L), and M is the amount of the sample to be measured (g).Calculation of relative odor activity value
Relative odor activity values (rOAVs) of volatile compounds of C. paliurus teas were calculated using formula (3) [Xue et al., 2022]:
where: rOAVi is the relative odor activity value of compound i, Ci is the content of the compound (μg/L), and Ti is the threshold of the compound (μg/L). Threshold values were taken from the literature [van Gemert, 2011; Yang et al., 2022a; Zhu et al., 2021].Statistical analysis
SIMCA P13 software (Umetrics, Umea, Sweden) and MEV 9.0 software (https://mev-tm4-org.caas) were used to analyze the main components and contributions of volatile metabolites in different tea processing, respectively. Three replicates were taken for each sample.
Principal component analysis (PCA) and orthogonal partial least squares discriminant analysis (OPLS-DA) were carried out with the Metware Cloud, a free online platform for data analysis (https://cloud.metware.cn). Significantly changed metabolites were selected according to variable importance in the project (VIP) (VIP≥1 and p<0.05) obtained by the OPLS-DA model. Duncan’s multiple test was applied to identify significant differences (p<0.05) among teas. Statistical analysis was performed using SPSS 18 (SPSS Inc., Chicago, IL, USA).
RESULTS AND DISCUSSION
Types of volatile metabolites in the C. paliurus teas
In this study, samples extracted from fixated and unfixated C. paliurus tea were analyzed by untargeted metabolomics. A total of 1,222 volatile compounds were detected, including 25 amines (2.05%), 95 alcohols (7.77%), 54 aromatics (4.42%), 29 phenols (2.37%), 10 nitrogen compounds (0.82%), 12 sulfur compounds (0.98%), 4 halogenated hydrocarbons (0.33%), 7 ethers (0.57%), 92 aldehydes (7.53%), 24 acids (1.96%), 309 terpenoids (25.29%), 85 hydrocarbons (6.96%), 99 ketones (8.1%), 172 heterocyclic compounds (14.08%), 199 esters (16.28%), and 6 others (0.49%).
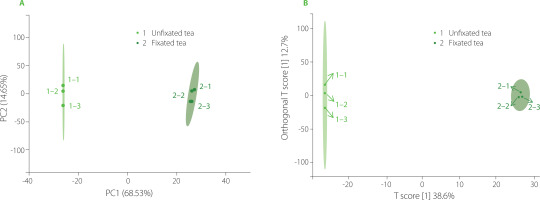
Using these 1,222 volatile metabolites, PCA was carried out on the C. paliurus teas prepared with and without the fixation step, and the resulting plot of PCA is shown in Figure 1A. The two processing methods were significantly distinguished from each other, and the percentage change of PC1 and PC2 interpretation was 83.18%; thus, these two principal components represented the main characteristics of the sample. Similarly, OPLS-DA, conducted with 1,222 volatile metabolites as independent variables and with fixated and unfixated teas as dependent variables, showed that there were large differences among sample groups, but small differences within groups (Figure 1B). The above results indicate that fixation was the key process in the processing of C. paliurus leaves, which had a great influence on the VOCs of the tea.
Figure 1
Score plots of principal component analysis (A) and orthogonal partial least squares discriminant analysis (B) based on volatile metabolites of fixated and unfixated Cyclocarya paliurus teas.
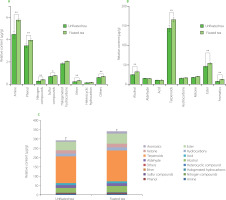
The numbers of volatile metabolites identified in fixated and unfixated teas and classified into different types are shown in Figure 2. Of the 1,222 VOCs, 1,196 metabolites were detected in C. paliurus tea prepared without the fixation step, while 1,193 volatile compounds were detected in tea obtained after leaf fixation. The main differences in the numbers of VOCs were found for terpenoids, heterocyclic compounds, amines, and phenols. Overall, there was little difference in numbers of compounds of each type between both tea variants, while the total VOC content in the C. paliurus tea obtained after fixation was significantly (p<0.05) higher than in the unfixated tea (Figure 3). Considering the different types of VOCs, contents of amines, phenols, nitrogen compounds, ethers, alcohols, terpenoids, esters, aromatics and others were extremely significantly higher (p<0.01) and the content of sulfur compounds was significantly higher (p<0.05) in the fixated tea (Figure 3A, B). Previous studies have found that the fixation process, due to the transient high temperature, can promote the hydrolysis, isomerization, substitution and redox reactions, and other physical and chemical thermal changes of the non-volatile compounds of green tea leaves, which, in turn, were the key prerequisite compounds for the VOC formation [Wang et al., 2021a]. The increase in the contents of VOCs of the fixated C. paliurus tea found in our study can be explained by a similar transformation.
Figure 2
Number of volatile metabolites of different classes identified in Cyclocarya paliurus teas produced with and without fixation.
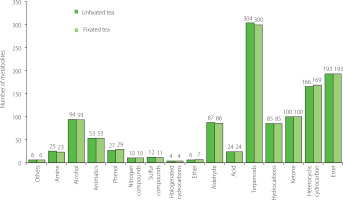
Figure 3
Contents of volatile metabolites of different classes (A and B) and total content of volatile metabolites (C) in Cyclocarya paliurus teas produced with and without fixation. Values in pairs (fixated and unfixated teas) marked with * differ significantly at p<0.05 and marked with ** differ significantly at p<0.01.
Individual volatile metabolites in the C. paliurus teas
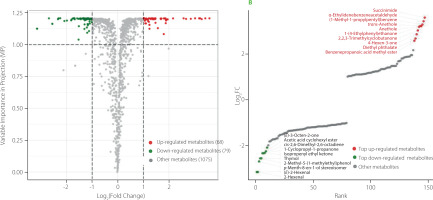
In order to elucidate the effect of fixation on the volatile metabolites in the processing of C. paliurus leaves, the differences of main metabolite contents in the key process of fixation were explored. According to the OPLS-DA screening criteria of metabolites: VIP ≥1 and fold change (FC) ≥2 or ≤0.5 [Geng et al., 2023], a total of 147 tea metabolites were screened, of which 68 were up-regulated and 79 down-regulated after fixation (Figure 4A). Among them, the top ten up-regulated were succinimide, α-ethylidenebenzeneacetaldehyde, (Z)-1-methoxy-4-(1-propenyl)benzene, trans-anethole, anethole, 1-(4-ethylphenyl)ethanone, 2,2,3-trimethylcyclobutanone, 4-hexen-3-one, diethyl phthalate, benzenepropanoic acid methyl ester, and the top ten down-regulated were 2-hexenal, (E)-2-hexenal, cis-p-menth-2-en-7-ol, 2-methyl-5-(1-methylethyl)phenol, thymol, isopropenyl ethyl ketone, 1-cyclopropyl-1-propanone, 2,6-dimethyl-2,6-octadiene, acetic acid cyclohexyl ester, and (E)-3-octen-2-one (Figure 4B).
Figure 4
Volcano plot of the volatile metabolites of fixated vs unfixated Cyclocarya paliurus teas with indicated compounds significantly up-regulated (red points) and down-regulated (green points) after fixation (A). Dynamic distribution of metabolites significantly differentiating teas with the top 10 metabolites that were up-regulated (red points) and down-regulated (green points) after fixation (B).
As shown in Table S1 in Supplementary Materials, 147 main metabolites included 13 aldehydes, 10 hydrocarbons, 15 heterocyclic compounds, 18 ketones, 18 esters, 9 aromatics, 18 alcohols, 32 terpenoids, 1 nitrogen compound,1 ether, 2 amines, 2 acids, 3 phenols, 3 sulfurs, and 2 others. In order to observe the differences between metabolite contents of fixated and unfixated teas in a more intuitive way, heat maps of 147 metabolites were obtained and they are show in Figure S1 in Supplementary Materials.
Terpenoids
Terpenoids, which were the most abundant among the VOCs of teas (Figure 2 and 3B) mainly included monoterpenoids, sesquiterpenoids and their derivatives. A total of 32 terpenes showed significant differences between tea variants, including 16 up-regulated and 16 down-regulated terpenes after fixation (Table S1, Figure S1). The first group included β-guaiene and β-pinene. Previously, these compounds were detected in black tea flavor profile [Yao et al., 2023]. One of the down-regulated terpenoid was α-terpineol, which was considered to be a non-flavor compound in beverages [Pérez-López et al., 2016], and has been identified as a key component of the stale odor of old green tea [Dai et al., 2020]. In our study, the content of l-α-terpineol in the C. paliurus tea decreased significantly as a result of fixation (Figure S1), indicating that the quality of the C. paliurus tea could be improved by this process. In addition, a previous study has shown that terpenoids are important characteristic compounds of C. paliurus [Shao et al., 2024], and volatile terpenoids are essential for the aroma quality of tea brews, with typical sweet, floral and woody flavors [Zhu et al., 2017].
Alcohols
There was a significant difference in the total alcohol content between the C. paliurus tea processed by fixation and the C. paliurus tea prepared without the fixation step (Figure 3B), of which 7 compounds were up-regulated and 11 compounds were down-regulated after fixation (Table S1, Figure S1). 2,3-Butanediol was included in the first group. This compound was also observed in the VOC profile of Huaguo tea [Lu et al., 2023], and Fuzhuan brick tea co-fermented with Moringa oleifera leaves [Li et al., 2023b]. (E)-2-Hexen-1-ol and (Z)-2-hexen-1-ol were identified to be more obvious in Huaguo tea [Lu et al., 2023]. After fixation, the down-regulation of some compounds that have adverse reactions to human inhalation was particularly obvious, such as 3-hydroxy-benzenemethanol, trans-2-undecen-1-ol, and prenol. Therefore, through the reduction of odor or harmful compounds, fixation offers also an important advantage to improve the quality of C. paliurus tea.
Esters
A total of 18 esters were detected in the fixated and unfixated teas, with 9 up-regulated and 9 down-regulated after fixation (Table S1, Figure S1). Previous studies have shown that high temperature and long-term treatment are conducive to the formation of ester volatiles [Zhou et al., 2023b], and fixation process results in a large amount of dissipation of low boiling point, grassy volatile esters [Ouyang et al., 2022]. These esters, together with ketones, aldehydes, and other metabolites, were responsible for chestnut-like, flowery, and fruity aromas of tea [Wang et al., 2021a]. Among up-regulated esters of C. paliurus tea, butyrolactone was determined (Figure S1). This compound was also found in the volatile compound profile of Congou black tea [Zhou et al., 2023a], green tea [He et al., 2023] and Dianhong Congou tea [Chen et al., 2022a].
Ketones
Ketones, which mainly contribute to woody and floral aromas [Liu et al., 2023], represent a common class of volatile compounds. In the study, it was found that 15 ketones were significantly down-regulated and only 3 were significantly up-regulated after fixation (Table S1, Figure S1). Among them, 2-heptanone down-regulated in the fixated tea, is commonly found in the VOCs of fruit and vegetable food products [Fella et al., 2022; Wang et al., 2021b] and is a flavor compound associated with fresh fruit aroma, with special banana aroma. 2-Heptanone can be formed by thermal β-oxidation of fatty acids, then decarboxylation [Valero et al., 2001], and can bind to proteins [Wang & Arntfield, 2015]. This may explain its decreasing content after high temperature treatment in our study. The three up-regulated ketones were 4-hexen-3-one, 2,2,3-trimethylcyclobutanone, and 1-(4-ethyl-phenyl)ethanone (Figure S1). 4-Hexen-3-one is an unsaturated aldehyde that was reported to occur in peels of Citrus aurantiifolia fruit [Sandoval-Montemayor et al., 2012], 1-(4-Ethylphenyl)ethanone was found in Rhus potaninii Maxim [Zhu et al., 2020], while 2,2,3-trimethylcyclobutanone has not been reported in literature concerning VOCs of plants.
Heterocyclic compounds
Heterocyclic compounds are also important aroma compounds, and occupy an important place in the VOCs of various varieties of tea [Guo et al., 2021]. A total of 15 heterocyclic compounds with significant differences were detected in C. paliurus tea variants, of which 8 were up-regulated and 7 were down-regulated after fixation (Table S1, Figure S1). The up-regulated compound, methylpyrazine, has nutty, roast and sweet aromas [Hu et al., 2021]; hence, its up-regulation endows the tea a unique nutty aroma. It was discovered in chestnut-like aroma green tea [Yang et al., 2022b]. 1H-Pyrrole-2-carboxaldehyde is a heat-induced compound (a previous study has shown that its content was higher in tomatoes obtained by heat pump drying [Jeyaprakash et al., 2020]). This may explain up-regulation of this compound after fixation in this study.
5-Ethylhydantoin, succinimide, 2-acetyl-3-ethylpyrazine, 1H-pyrrole-2,5-dione, and (3R,6S)-2,2,6-trimethyl-6-vinyltetrahydro-2H-pyran-3-ol were the five significantly up-regulated heterocyclic compounds (Figure S1) that have not been reported in the volatility studies of other species, and can be used as characteristic compounds of C. paliurus tea obtained through fixation. Among the down-regulated heterocyclic compounds, betahistine, which is used in western medicine for the treatment of cardiovascular disease, was found in C. paliurus leaves for the first time. 2-n-Heptylfuran is only produced in a thermal reaction at 14°C and is associated with the flavor of cocoa and roasted nuts [Li et al., 2023a]. However, the temperature of the fixation reached 170–180°C; hence, the content of this compound in tea decreased after processing. 1-Methyl-2-pyrrolidinone, 4-(N-nitroso-N-methylamino)-1-(3-pyridyl)-1-butanone, 2-furanpropanoic acid ethyl ester, dihydro-5-pentyl-2(3H)-furanone, and 1,5,6-trimethyl-azacyclohexan-3-one were the five down-regulated heterocyclic compounds (Figure S1) that have not been reported in the volatile compound profile of other species.
Aldehydes
Aldehydes with low odor threshold [Culleré et al., 2011] can provide special flavor for C. paliurus tea. In addition, they perform a variety of biological functions, e.g., cinnamaldehyde and transcinnamaldehyde showed antifungal and antibacterial activities [Gu et al., 2024; Mu et al., 2023]. The first of the mentioned aldehydes was up-regulated after fixation in our study (Table S1, Figure S1). Generally, in the C. paliurus teas, 6 aldehydes were significantly up-regulated and 7 others were significantly down-regulated after fixation. Up-regulated 2,4-dimethylbenzaldehyde was also found in a previous study as an odor active compound of the sour bamboo shoot fermentation broth [Long et al., 2023]. 4-Isopropylcyclohexa-1,3-dienecarbaldehyde and 8-isopropyl-1-methyltricyclo[4.4.0.02,7]dec-3-ene-3-carbaldehyde were two significantly up-regulated aldehydes that have not been reported in studies of VOSs of other species, and can be used as the characteristic compounds derived from C. paliurus tea.
2-Hexenal and (E)-2-hexenal are common volatile aldehydes, which have been recognized as signature characteristic compounds in tea processing [Rong et al., 2023; Yang et al., 2023]. Similarly, 2-undecenal and (E)-2-undecenal (the cis-trans isomers) are also typical volatile aldehydes sensitive to heating [Zhou et al., 2021], and this is the first time when they had been detected in the VOCs of C. paliurus leaves. Studies have shown that (E,E)-2,4-nonadienal appeared to be mainly responsible for the stale odor in green tea [Liu et al., 2023], which indicated that the significant down-regulation of (E,E)-2,4-nonadienal could increase the flavor quality of C. paliurus fixated tea. (E)-2-Decenal was the main aroma compound in Angelica keiskei tea [Rong et al., 2021], presenting typical flower, fruity, and sweet flavor. The significant down-regulation of this compound after fixation in our study, may be related to its properties. After heating, it can continue to oxidize to form volatile substances with short carbon chains [Wang et al., 2023]. 5-(Propan-2-yl)bicyclo[3.1.0] hex-2-ene-2-carbaldehyde has not yet been reported in the VOCs of C. paliurus leaves.
Hydrocarbons
Weakly polar hydrocarbons are also characteristic flavor compounds in teas [Guo et al., 2021]. The 10 individual hydrocarbons (Table S1, Figure S1) included down-regulated after fixation olefins (4 compounds) and alkanes (2 compounds), as well up-regulated alkenes (3 compounds) and alkane (1 compound). Among them, 2,6-dimethyl-2,6-octadiene is produced during the thermal decomposition of glycosides [Hattori et al., 2004]. Heating at 130°C/10 min + 110°C/30 min was conducive to its formation in black tea [Yang et al., 2020]. In this study, the fixation temperature was higher (170°C–180°C); hence, its content decreased significantly. Albene has not been found in the VOCs of other species, especially tea, except in the roots of yarrow [Kindlovits et al., 2018]. In addition, other up-regulated olefins and alkanes are rare in the study of volatile compound profile, and can be used as the characteristic compounds of C. paliurus tea for further study.
Aromatics
Contents of nine aromatics in C. paliurus tea variants showed significant differences, of which six were up-regulated after fixation (Table S1, Figure S1). Surprisingly, anethole and transanethole, which exists in fennel seeds [Odeh & Allaf, 2017], were found in C. paliurus tea, and they were significantly up-regulated after fixation. Due to their weet, herbaceous, and smooth odor profile, anethole and trans-anethole exhibited a comparatively high rOAV, indicating their crucial role in creating distinctive flavors and making them the primary contributor to the overall flavor of Yunan and Fujian tea [Kfoury et al., 2018]. Estragole, a volatile phenylpropanoid contained in a variety of edible herbs [Gross et al., 2009; Yamani et al., 2014], was suspected to be carcinogenic and genotoxic, according to the European Union Committee on Herbal Medicinal Products (https://www.ema.europa.eu/en/committees/committee-herbal-medicinalproducts-hmpc). Therefore, further studies are needed on a safe daily intake of C. paliurus herbal tea. The 1,2-dihydro-1,1,6-trimethyl-naphthalene was found as a key aroma compound of Pu-erh tea (ripe tea) [Wang et al., 2022]. It was also significantly up-regulated in C. paliurus tea after fixation. The other two up-regulated aromatics, i.e., 1,2,3,4-tetrahydronaphthalene and 2-phenylpropenal, have not been reported in any tea volatile compound profile The aromatics down-regulated after fixation were 2-methoxy-4-vinylphenol, (1-methyl-1-propylpentyl) benzene, and 1,1’-(1,3-propanediyl)bis-benzene. Among them, 2-methoxy-4-vinylphenol has the clove-like flavor, which has been reported to be a landmark compound of canned black tea after heating [Kumazawa & Masuda, 2001]. The fixation temperature was significantly higher than that of black tea sterilization; thus, the changes of 2-methoxy-4-vinylphenol caused by the two treatments were inconsistent.
Other compounds
In addition to the volatile compounds mentioned above, the other up-regulated volatile compounds of teas after fixation included carbonochloridodithioic acid methyl ester, ethanediamide, benzoic acid, 2-(1-methylpropyl)phenol, cis-p-menth-2-en-7-ol, dipropyl disulfide, (Z)-1-methoxy-4-(1-propenyl)benzene, benzyl nitrile, and ethyl 1-methylethyl disulfide (Table S1, Figure S1). Among them, benzoic acid has a floral and fruity flavor, and its content was also significantly up-regulated after green tea and yellow tea fixation [Wen et al., 2023], which is consistent with our findings. Benzyl nitrile has a pungent odor [Li &Wang, 2020], which has been identified as a key marker for the floral odor of Fenghuang Dancong tea [Qin et al., 2023]. The significantly up-regulated cis-p-menth-2-en-7-ol, has been previously found in Ephedra sinica Stapf. [Miyazawa et al., 1997].
Four volatiles of C. paliurus teas were down-regulated after fixation included butanoic anhydride, N-phenylacetamide, (E)-2-hexenoic acid, and 2-(1,1-dimethylethyl)phenol. (E)-2-Hexenoic acid, with a fruity, herbal odor, has also been found in ferns [Fons et al., 2010].
Aroma compounds of C. paliurus teas
Although a variety of volatile components were screened in different processed products of C. paliurus tea, not all of the volatile compounds were the material basis for the odor difference between fixated and unfixated C. paliurus teas. Therefore, the rOAV method was used to determine the contribution of volatile components to the overall odor of teas, and then to identify the key aroma compounds. In general, rOAV≥1 indicates that the compound has a direct contribution to the sample flavor, and 0.1≤rOAV<1 can be considered to have an important modification effect on the aroma of the sample [Huang et al., 2022; Xue et al., 2022]. As shown in Table 1, there were 46 volatile compounds with rOAV value greater than 0.1 in the unfixated C. paliurus tea, among which 22 volatile compounds had rOAV greater than 1. In the fixated C. paliurus tea, there were 44 volatile compounds with rOAV greater than 0.1, and 25 volatile compounds with rOAV greater than 1.
Table 1
Threshold, odor description and relative odor activity value (rOAV) of volatile compounds identified in Cyclocarya paliurus tea produced with and without fixation.
| Compound | Class | Odor | Threshold (μg/L) | rOAV* | |
|---|---|---|---|---|---|
| Unfixated tea | Fixated tea | ||||
| 1-Hexen-3-one | Ketones | Cooked, vegetable, metallic | 2×10−5 | 25,371±1826 | 13,291±74 |
| 3-Octen-2-one | Ketones | Earthy, spicy, herbal, sweet, mushroom, hay, blueberry | 3×10−5 | 5,419±607 | 1,810±119 |
| 2-Methylisoborneol | Terpenoids | Earthy, musty | 0.0005 | 551±27 | 273.8±8.3 |
| Anethole | Aromatics | Sweet, exotic, flowery, stewed | 0.015 | 21.3±1.4 | 212.8±7.8 |
| (E,E)-2,4-Nonadienal | Aldehydes | Fatty, melon, waxy, green, violet, leafy, cucumber, tropical, fruity, chicken | 0.0002 | 272±12 | 143.4±4.3 |
| (E)-2-Undecenal | Aldehydes | Fresh, fruity, citrus, orange peel | 0.0008 | 296±16 | 143.1±4.2 |
| Heptanoic acid methyl ester | Esters | Sweet, fruity, green, orris, waxy, floral, berry | 0.004 | 39.0±2.7 | 88.6±5.7 |
| (E)-6,10-Dimethyl-5,9-undecadien-2-one | Ketones | Fresh, green, fruity, waxy, rose, woody, magnolia, tropical | 0.01 | 162.5±8.1 | 68.3±2.4 |
| trans-Anethole | Aromatics | Sweet, anisic, licorice, mimosa | 0.057 | 5.62±0.37 | 56.0±2.1 |
| (E)-2-Hexenal | Aldehydes | Green, grassy | 0.0031 | 388±193 | 47.8±2.1 |
| Acetic acid cyclohexyl ester | Esters | Fruity, sweet, musty, ethereal | 0.0016 | 117.6±9.0 | 26.6±1.1 |
| β-Pinene | Terpenoids | Dry, woody, resinous, pine, hay, green | 0.14 | 7.32±0.46 | 16.15±0.50 |
| 1,2-Dihydro-1,1,6-trimethyl-naphthalene | Aromatics | Licorice | 0.0025 | 5.19±0.23 | 12.16±0.40 |
| 2-Undecenal | Aldehydes | Fresh, fruity, orange, peel | 0.01 | 23.1±1.3 | 11.17±0.33 |
| 2-Hexenal | Aldehydes | Sweet, almond, fruity, green, leafy, apple, plum, vegetable | 0.017 | 71±35 | 8.72±0.38 |
| 2-Methoxy-4-vinylphenol | Aromatics | Spicy, raisin | 0.003 | 15.62±0.60 | 8.02±0.34 |
| (E)-2-Decenal | Aldehydes | Waxy, fatty, earthy, green, cilantro, mushroom, aldehydic, fried, chicken, fatty, tallow | 0.005 | 14.21±0.68 | 7.28±0.37 |
| 6,6-Dimethyl-bicyclo[3.1.1]hept-2-ene-2-methanol | Terpenoids | Woody, minty | 0.007 | 8.20±0.45 | 3.56±0.23 |
| Acetic acid 4-methylphenyl ester | Esters | Narcissus, phenol, animalic | 0.025 | 0.766±0.069 | 2.941±0.090 |
| Diethyl phthalate | Esters | Bitter | 0.33 | 0.384±0.033 | 2.65±0.25 |
| 3-Phenyl-2-propenal | Aldehydes | Sweet, spicy, aldehydic, aromatic, balsamic, cinnamyl, resinous, honey, powdery | 0.024 | 0.58±0.11 | 2.392±0.099 |
| Dipropyl disulfide | Sulfur compounds | Sulfury, earthy, burnt, green, onion | 0.0195 | 0.495±0.038 | 1.348±0.051 |
| α-Ethylidenebenzeneacetaldehyde | Aldehydes | Sweet, narcissus, cortex, beany, honey, cocoa, nutty, radish | 0.5 | 0.110±0.007 | 1.271±0.052 |
| Estragole | Aromatics | Sweet, sassafrass, anisic, spice, green, herbal, fennel | 0.035 | 0.414±0.025 | 1.188±0.032 |
| Eucalyptol | Terpenoids | Eucalyptus, herbal, camphor, medicinal | 0.015 | 0.365±0.032 | 1.040±0.058 |
| α-Terpineol | Terpenoids | Pine, iris, teil | 0.3 | 2.28±0.18 | 0.964±0.032 |
| (E)-2-Hexen-1-ol | Alcohols | Fresh, green, leafy, fruity, unripe banana | 0.1 | 0.330±0.015 | 0.897±0.005 |
| (Z)-2-Hexen-1-ol | Alcohols | Green, cortex, leafy, beany, nasturtium, herbal, soapy, aldehydic, narcissus, phenol | 0.1 | 0.330±0.015 | 0.897±0.005 |
| Dihydro-5-pentyl-2(3H)-furanone | Heterocyclic compounds | Coconut, woody | 0.0079 | 2.42±0.27 | 0.892±0.067 |
| l-α-Terpineol | Terpenoids | Lilac, floral, terpenic | 0.33 | 2.07±0.16 | 0.876±0.030 |
| Methylpyrazine | Heterocyclic compounds | Nutty, cocoa, roasted, chocolate, peanut, green | 0.06 | 0.306±0.037 | 0.689±0.020 |
| Prenol | Alcohols | Fruity, green, lavender | 0.25 | 1.37±0.10 | 0.664±0.020 |
| α-Methyl-benzenemethanol | Alcohols | Fresh, sweet, gardenia, hyacinth | 0.479 | 0.238±0.031 | 0.621±0.023 |
| (1S)-6,6-Dimethyl-2-methylene--bicyclo[3.1.1]heptane | Terpenoids | Dry, woody, fresh, pine, hay, green, resinous | 4.16 | 0.246±0.016 | 0.544±0.017 |
| 4-Methyl-1-(1-methylethyl)-bicyclo[3.1.0] hex-3-en-2-one | Terpenoids | Minty, pungent | 0.75 | 0.158±0.009 | 0.469±0.034 |
| 3,7-Dimethyl-1,5,7-octatrien-3-ol | Alcohols | Mouldy | 0.11 | 0.875±0.016 | 0.421±0.066 |
| Hotrienol | Alcohols | Sweet, tropical, ocimene, fennel, ginger, myrcene | 0.11 | 0.875±0.016 | 0.421±0.066 |
| 4-Methylene-1-(1-methylethyl)--bicyclo[3.1.0]hexane | Terpenoids | Woody, terpene, citrus, pine, spice | 0.98 | 0.144±0.013 | 0.360±0.010 |
| 4-Methyl-1-(1-methylethyl)-bicyclo[3.1.0] hex-2-ene | Hydrocarbons | - | 0.98 | 0.118±0.008 | 0.260±0.010 |
| Isopentyl hexanoate | Esters | Fruity, banana, apple, pineapple, green | 0.32 | 0.53±0.10 | 0.251±0.005 |
| (E)-3,7,11-Trimethyl-1,6,10-dodecatrien-3-ol | Terpenoids | Floral, green, citrus, woody, waxy | 0.25 | 0.478±0.0145 | 0.241±0.037 |
| 2-(1,1-Dimethylethyl)phenol | Phenols | - | 0.05 | 0.421±0.040 | 0.202±0.030 |
| (Z)-3-Hexen-1-ol benzoate | Esters | Fresh, green, leafy, floral, orchid, balsamic, fatty | 0.5 | 0.046±0.011 | 0.183±0.009 |
| (E)-2-Hexenoic acid | Acids | Powerful, fruity, sweet, warm, herbal | 1.9 | 0.409±0.019 | 0.175±0.011 |
| 3-Hexanone | Ketones | Sweet, fruity, waxy, rummy, grape | 0.041 | 0.396±0.030 | 0.148±0.041 |
| 1,2,3,4-Tetrahydronaphthalene | Aromatics | - | 0.05 | 0.054±0.006 | 0.135±0.009 |
| Benzyl nitrile | Nitrogen compounds | - | 1.2 | 0.031±0.001 | 0.105±0.004 |
| Acetic acid 2-ethylhexyl ester | Esters | Earthy, herbal, humus, undergrowth | 0.047 | 0.162±0.017 | 0.058±0.003 |
| Thymol | Terpenoids | Herbal, thyme, phenol, medicinal, camphor | 0.188 | 0.220±0.070 | 0.040±0.003 |
Many identified ketones, aldehydes, terpenes and other compounds in the tea produced by the two processing technologies had a high content and a relatively low threshold; hence, they contributed a lot to the overall flavor of the tea. The compound that contributed the most was 1-hexen-3-one (ketone), which mainly presents a cooked, vegetable, and metallic aroma. Followed by 3-octen-2-one, also a ketone, mainly presents earthy, spicy, herbal, sweet, mushroom, hay, and blueberry aroma. In addition, 5 terpenoids (2-methylisoborneol, 6,6-dimethyl-bicyclo[3.1.1]hept-2-ene-2-methanol, β-pinene, α-terpineol and l-α-terpineol), 6 aldehydes (2-hexenal, (E)-2-undecenal, (E,E)-2,4-nonadienal, (E)-2-hexenal, 2-undecenal, (E)-2-decenal), 4 aromatics (anethole, 2-methoxy-4-vinylphenol, trans-anethole, and 1,2-dihydro-1,1-6-trimethyl-naphthalene), 2 esters (acetic acid cyclohexyl ester and heptanoic acid methyl ester), 1 heterocyclic compound (dihydro-5-pentyl-2(3H)-furanone), 1 alcohol (prenol), and 1 ketone ((E)-6,10-dimethyl-5,9-undecadien-2-one) in the unfixated C. paliurus tea are its characteristic aroma substances. Green and fruity were the two main aromas, which were presented by a variety of substances. In the fixated C. paliurus tea, 8 aldehydes ((E,E)-2,4-nonadienal, (E)-2-undecenal, (E)-2-hexenal, 2-undecenal, 2-hexenal, (E)-2-decenal, 3-phenyl-2-propenal, and α-Et hylidenebenzeneacetaldehyde), 5 aromatics (anethole, transanethole, 1,2-dihydro-1,1,6-trimethyl-naphthalene, 2-methoxy-4-vinylphenol, and estragole), 4 terpenoids (2-methylisoborneol, β-pinene, 6,6-dimethyl-bicyclo[3.1.1]hept-2-ene-2-methanol, and eucalyptol), 4 esters (heptanoic acid methyl ester, acetic acid cyclohexyl ester, acetic acid 4-methylphenyl ester, and diethyl phthalate), 1 sulfur compound (dipropyl disulfide), and 1 ketone ((E)-6,10-dimethyl-5,9-undecadien-2-one) were its characteristic aroma compounds. Green and sweet were the two main aromas, which were presented by a variety of compounds.
CONCLUSIONS
Herein, the effect of fixation on the volatile compounds of C. paliurus tea was investigated and analyzed comprehensively for the first time. A total of 1,222 volatile metabolites were detected and 147 individual volatile metabolites were screened. Fixation had a strong effect on the content of volatile compounds in C. paliurus tea. After fixation, the content of amines, phenol, nitrogen, ether, alcohols, terpenoids, ester, aromatics increased significantly. The difference of metabolites before and after fixation indicates that fixation was a key step in C. paliurus tea processing. In addition, the study also found a batch of unique volatile compounds of C. paliurus tea and key aroma compounds, which present mainly green and sweet odor. The results of this study provided a new theoretical basis for the development of processing technology of C. paliurus leaves into tea, which could be used for quality control during the processing of C. paliurus leaves. In the follow-up study, we will investigate the potential effects of amino acids, lipids etc. added during the fixation step on the flavor characteristics of C. paliurus tea.

