INTRODUCTION
Chenopodium quinoa Wild. is an emerging crop worldwide, cultivated for over 7,000 years. It is characterized by its ability to grow in different marginal environments [Choukr-Allah et al., 2016], with marked resistance to various abiotic stresses, including salinity, drought, and frost [Jacobsen et al., 2003; Nazih et al., 2024].
C. quinoa seeds are distinguished by their high nutritional value and bioactive properties [Nowak et al., 2016; Vega-Gálvez et al., 2010]. They are rich in protein (9.1 to 15.7 g/100 g), lipids (4.0 to 7.6 g/100 g) and crude fiber (7.0 to 14.1 g/100 g), and their average carbohydrate content is 58 g/100 g [Pathan & Siddiqui, 2022]. Quinoa seed proteins contain all the essential amino acids and have a high efficiency ratio. These seeds are also rich in minerals, including potassium, calcium, magnesium, and iron [Pathan & Siddiqui, 2022]; tocopherols (7.29 mg/100 g) [Carciochi et al., 2016]; and phenolic compounds (71.7 mg/100 g) with high antioxidant activity [Alvarez-Jubete et al., 2010]. Other important phytochemicals of quinoa seeds are saponins with contents between 1.41 and 2.03 g/100 g [Mhada et al., 2020]. It should be emphasized, however, that the nutritional and bioactive composition of C. quinoa seeds depends on the plant genotype and the environmental conditions in which it grows [Granado-Rodríguez et al., 2021a,b]. From a nutritional point of view, regular consumption of quinoa seeds in the diet elicits certain health benefits and can reduce the risk of development of cardiovascular disease, obesity and diabetes [Lan et al., 2023]. Quinoa is also a good alternative for patients suffering from celiac disease as it is gluten-free [Alvarez-Jubete et al., 2010].
Due to its precious qualities, quinoa, with its multiple uses, certainly has a great future, especially in arid and semi-arid areas severely affected by climate impacts, as is the case in several marginal regions of Morocco. In this context, several studies have shown that germination enhances the nutritional value of quinoa by modifying its composition [Omary et al., 2012; Suárez-Estrella et al., 2020]. Bhinder et al. [2021] showed that germination can modify protein content, starch functionality, and bioactive compound profile, enhance the availability of certain minerals such as copper and zinc, and reduce antinutrient levels, including saponins and phytic acid. Additionally, Ramos-Pacheco et al. [2024] observed a notable improvement in proteins, lipids, ash, and fiber and a reduction in carbohydrate contents of quinoa seeds as a result of germination. They also noted increases in phosphorus, iron, manganese, and potassium, as well as in total phenolic and total flavonoid contents and antioxidant capacity. These modifications could be due to the activation of endogenous enzymes, which reduce antinutrients and improve nutrient profile, and antioxidant potential [Darwish et al., 2021]. However, most of these studies focused on shorter germination periods (up to 72 h) and did not fully investigate the variations in bioactive compounds, especially tocopherols. Therefore, the current study aims to evaluate the effect of prolonged germination (up to 120 h) on the nutritional and bioactive compound compositions of C. quinoa seeds and to determine the optimal germination time that enhances the content of valuable compounds, in order to find alternatives for patients with gluten intolerance or celiac disease and individuals seeking highly nutritious food. In addition, the current study provides new insights into the effect of germination on the contents of macronutrients, minerals, and bioactive compounds of quinoa grown and harvested in the eastern region of Morocco.
MATERIALS AND METHODS
Plant material
To conduct this study, fresh-matured C. quinoa seeds of the certified Titicaca cultivar were harvested from a farm in Berkane province in the eastern region of Morocco. Titicaca is a Danish quinoa variety developed by the University of Copenhagen. It is known for its early maturity, tolerance to abiotic stress, and adaptability to marginal soils. Its fruit is an achene containing round seeds about 2 mm in diameter.
Germination of C. quinoa seeds
The process of germination of quinoa seeds was carried out according to the methodology of Aguilar et al. [2019], with slight modifications. A total of 720 g of C. quinoa seeds (120 g per germination time, including the control) were soaked in 3.6 L of distilled water (600 mL per batch) for 24 h. After soaking, the seeds were placed in plastic boxes (three boxes for each germination time) with filter paper wetted with distilled water to keep moisture conversing. The boxes were then transferred to a growth chamber (Memmert GmbH, Schwabach, Germany), where the temperature, relative humidity, and light/dark cycle were 25°C, 70%, and 16/8 h respectively. The soaked seeds (control, germination time – 0 h) and seeds after 24, 48, 72, 96 and 120 h of germination were dried in a forced-air oven (Pol-Eko Aparatura, Wodzisław Śląski, Poland) at 40°C for 24 h to inhibit the activity of hydrolytic enzymes such as amylases, proteases and phytases without significantly altering heat-sensitive nutrients [Guardianelli et al., 2022], then crushed with a mill and sifted with a 500 µm sieve to obtain the powders, which were stored at −20°C until analysis of their nutrient and bioactive compound profiles.
Water activity measurement
Water activity (aw) of powders from sprouted quinoa seeds was measured using an AW meter (Steroglass, Perugia, Italy). Calibration was performed with pure water of aw=1. Samples weighing 1 g were used for the aw reading [Ligarda-Samanez et al., 2022].
Dry matter determination
The dry matter content of powdered quinoa seeds after germination and drying was determined using the AOAC International method 934.01 [AOAC, 2005]. The samples (1 g) were heated at 105°C until their mass reached a constant value.
Protein content determination
To determine the total nitrogen content of the sprouted quinoa seeds by the Kjeldahl method [AFNOR, 2002], 1 g of powder was weighed and placed in a mineralization tube containing 2 g of catalyst (K₂SO₄, CuSO₄×5H₂O, Se), 10 mL of 30% hydrogen peroxide and 20 mL of 98% sulfuric acid. The heating protocol involved a progressive temperature: 45 min at 190°C, 45 min at 290°C until carbonization, and 3 h at 420°C until a lipid liquid appears. After mineralization, 50 mL of distilled water and 80 mL of 40% sodium hydroxide were added for distillation, followed by collection in 50 mL of 4% boric acid and titration with 0.2 N sulfuric acid. A blank sample was treated in the same way for each series. The total nitrogen content (N, %) was calculated using Equation (1):
where: V is volume of H2SO4 used for the sample titration in mL, V0 is volume of H2SO4 used for the blank titration in mL, NA is acid solution normality, and E is sample weight in g.Protein content was obtained by multiplying N×6.25 [Nascimento et al., 2014; Zhou et al., 2023] and expressed in g/100 g of dry matter (DM) of sprouted seeds.
Lipid content determination
A Soxhlet extractor (Gerhardt, Konigswinter, Germany) with n-hexane as a solvent was used for lipid determination according to the AOAC International method 920.39 [AOAC, 1990]. The procedure consisted of placing 40 g of sprouted quinoa seed powder in a cellulose cartridge. The cartridge was then placed in a ground flask containing 150 mL of n-hexane. The flask was fitted with a heating mantle and heated for 6 h. The solvent was then evaporated by distillation using a rotary evaporator (Hahn Vapor, Gimpo, South Korea). Finally, the flask containing the resulting lipids was weighed. The lipid content of sprouted seeds was expressed in g/100 g DM.
Crude fiber determination
The crude fiber was determined according to the AOAC International method 988.15 [AOAC, 1990] using a Fibertech system (VELP Scientifica, Usmate Velate, Italy). The sprouted quinoa seed powder was decarbonated and degreased with 100 mL of 1.25 N sulfuric acid and 100 mL of 1.25 N sodium hydroxide solutions. The residue obtained was then separated by filtration through a glass filter. The crucibles containing the residue were then washed and dried in an oven at 103°C for 12 h to obtain the dry weight (Dw) and then incinerated in a muffle furnace at 550°C for 5 h to obtain the ash weight (Aw). The crude fiber content (CF) was calculated according to the Equation (2) and expressed in g/100 g DM of sprouted seeds:
where: T is tare weight and Sw is sample weight.Ash content determination
The ash content was determined by the AOAC International method 942.05 [AOAC, 2005]. The powders weighing 1 g were incinerated in a muffle furnace (Nabertherm GmbH, Lilienthal, Germany) at 650°C for 8 h. The ashed samples were weighed, and ash content of sprouted seeds was expressed in g/100 g DM.
Carbohydrate content estimation
Carbohydrate content of sprouted quinoa seeds was estimated by difference according to Equation (3) [Abedin et al., 2022]:
The results were expressed in g/100 g DM of sprouted seeds.
Energy value estimation
The energy value of the sprouted quinoa seed powders was calculated using Equation (4) [FAO, 2003]:
Results were expressed in kcal/100 g DM.
Mineral composition analysis
The method described by Granado-Rodríguez et al. [2021b], with some modifications, was used to determine the content of K, Mg, Ca, Fe, Zn, Cu, and Mn in sprouted quinoa seeds. Powders weighing 2 g were placed in a muffle furnace at a temperature of 650°C for 4 h. The ash formed was dissolved with 3 mL of concentrated hydrochloric acid (37%) and heated in a boiling water bath until the ash was completely dissolved. The volume was made up of 100 mL with pure water, and the solution was analyzed using an atomic absorption spectrophotometer (PerkinElmer, Waltham, MA, USA). The results were expressed in g/kg DM of sprouted seeds.
Determination of total phenolic content and tot al flavonoid content
The powders were extracted according to the method proposed by Ollivier et al. [2004]. The sample was weighed (500 mg) and added to 3 mL of a mixture of methanol and distilled water (80:20, v/v) in Eppendorf tubes. The tubes were vortexed at 1,500 rpm for 15 min and then centrifuged at 1,130×g for 15 min. The supernatant was collected into a 10 mL flask, and the extraction was repeated three times. Finally, the samples were spiked with methanol and stored in a freezer at −20°C until analysis.
The total phenolic content was determined with a Folin-Ciocalteu reagent according the method described by Joy Ujiroghene et al. [2019], with slight modifications. The extract (2 mL) was mixed with 5 mL of 10% Na2CO3, 1 mL of the Folin-Ciocalteu reagent, and 5 mL of distilled water. This mixture was incubated in the dark for 30 min. Absorbance readings were then taken using a spectrophotometer (PG Instruments Ltd, Lutterworth, United Kingdom) at a wavelength of 750 nm. The results were expressed as mg gallic acid equivalent (GAE) per 100 g DM of sprouted seeds.
The total flavonoid content was quantified using the aluminum chloride colorimetric method described by Suárez-Estrella et al. [2020], with minor modifications. Volume of 1 mL of 2% AlCl3 was added to 1 mL of the extract. After shaking, the mixture was incubated for 10 min. Its absorbance was then read with a spectrophotometer (PG Instruments Ltd) at a wavelength of 430 nm. The results were expressed as mg quercetin equivalent (QE) per 100 g DM of sprouted seeds.
Determination of saponin content
The method used to extract saponins from sprouted quinoa seed powders was the one described by Rafik et al. [2021], which involved a Soxhlet extraction with n-hexane, repeated three times, to delipidate the powder. Next, 5 g of the delipidated powder was mixed with 50 mL of ethanol and stirred for 30 min. The mixture was then filtered, and the filtrate collected was made up to 50 mL with ethanol. Then, 2 mL of a reagent (mixture of glacial acetic acid and concentrated sulfuric acid (1:1, v/v), was added to 250 µL of the extract, and the mixture was vortexed and incubated at 60°C for 30 min in a water bath (Bunzen, Madrid, Spain) [Torrez Irigoyen & Giner, 2018]. After incubation, the mixture was cooled in an ice bath. The absorbance was measured using a spectrophotometer (PG Instruments Ltd) at 527 nm. Saponin (CAS No. 8047-15-2), purchased in Solvachim (Casablanca, Morocco), was used as a reference. The results were expressed as g saponin standard equivalent per 100 g DM of sprouted seeds.
Tocopherol analysis
To determine tocopherols, 200 mg of oil, extracted from quinoa powder by solid-liquid extraction (Soxhlet) and previously filtered with a 0.45 μm filter, was dissolved in 1 mL of methanol. The mixture was then vortexed at 1,500 rpm for 2 min. Finally, the sample was injected into a column connected to a high-performance liquid chromatography (HPLC) system (Agilent Technologies, Waldbronn, Germany) with fluorescence detector (FLD) for tocopherol analysis. The chromatographic separation was performed on a Poroshell 120 EC-C18 column (4.6×150 mm, 4 µm; Agilent Technologies) in an isocratic elution mode. The mobile phase was a mixture of acetonitrile and methanol (50:50, v/v) (both solvents of HPLC grade), with a flow rate of 1 mL/min and an injection volume of 20 µL. Detection was performed at 290 nm for excitation and 330 nm for emission. A calibration curve was plotted using α-tocopherol, β-tocopherol, γ-tocopherol and δ-tocopherol standards. The results were expressed as µg/g of oil.
Statistical analysis
Three replicates were performed for each treatment (germination time) and three samples (one from each replicate) were analyzed by each method. Means and standard deviations were calculated. One-way analysis of variance (ANOVA) was used to determine the effect of germination time (factor) on quinoa composition using IBM SPSS Statistics 25.0 (IBM Corp., Armonk, NY, USA). The post hoc Tukey’s test was used to compare means and determine significant differences between samples. Differences were considered as significant at p≤0.05. The graph was generated using Excel software for Microsoft 365 MSO, version 2405 (Microsoft Corp., Redmond, WA, USA).
RESULTS AND DISCUSSION
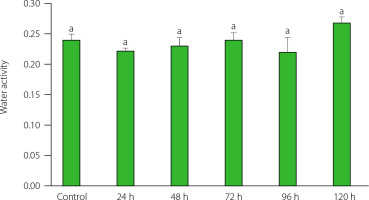
Effect of germination time on water activity
Water activity of powders obtained from ungerminated and germinated quinoa seeds varied between 0.22 and 0.27, with insignificant (p>0.05) differences between powders from germinated and control seeds as well as between powder from seeds sprouted for different times (Figure 1). This slight change in aw indicated that the powders did not present a risk of proliferation of microorganisms [Pellegrini et al., 2018] and can be used as a safe ingredient. Our results were consistent with those reported by Ramos-Pacheco et al. [2024], who found values of aw<0.3 in all germinated and ungerminated quinoa seed powders.
Effect of germination time on macronutrient content
Protein content
The protein content of sprouted quinoa seeds varied between 15.73 and 23.64 g/100 g DM (Table 1). Compared to the control (17.55 g/100 g DM), a significant (p≤0.05) decrease was observed in the seeds sprouted for 24 h. However, an important increase (p≤0.05) was observed in the seeds sprouted for 48–120 h, with the highest protein content determined in the seeds germinated for 96 h (increase of 6.09 g/100 g DM compared to the control). This remarkable augmentation could be explained by the synthesis of new amino acids and the loss of carbohydrates through seed respiration during germination [Bertazzo et al., 2011]. In addition, Pilco-Quesada et al. [2020] explained the augmentation in protein content during germination by biological synthesis and the mobilization of nutrient reserves. Bewley et al. [2013] reported that during the first three days of germination, amino acid content increased, leading to an increase in protein content. In the same context, Jimenez et al. [2019] estimated that 2 to 3 days after imbibition, proteolytic enzymes hydrolyzed proteins into peptides and amino acids, increasing the bioavailability of nutrients. Our study results are close to those of Aguilar et al. [2019], who observed that C. quinoa seeds (Negra Collana variety) displayed a maximum increase of 8% in protein content after 48 h of germination. Similarly, Thakur et al. [2021] noted a considerable improvement in protein content from 14.94% (control) to 17.88% after 72 h of germination of quinoa seeds. Pilco-Quesada et al. [2020] also observed an enhancement in protein from 9.6 to 26.0% after 72 h of germination. Guardianelli et al. [2022] also confirmed a positive correlation between germination time and protein content.
Table 1
Macronutrient content and energy value of quinoa seeds sprouted for different times.
Our findings, in accordance with literature data, suggest that germination is an effective strategy for enhancing the protein content of quinoa, making it a promising approach for improving the nutritional value of quinoa-based food products.
Lipid content
The lipid content of sprouted seeds ranged from 3.38 to 5.05 g/100 g DM, with insignificant (p>0.05) variation observed between germinated and control seeds (Table 1). However, the continuous significant (p≤0.05) increase in lipid content was found from 24 to 72 h of processing. This minor uptick in lipid content could be attributed to the abundance of fatty acids released from triglycerides or phospholipids by lipolytic enzymes during germination [Obizoba & Atii, 1991]. On the other hand, during germination, seeds use fatty acids to produce sucrose via gluconeogenesis, which is an energy source necessary to complete growth [Lan et al., 2023; Nelson et al. 2013].
Our study results do not agree with those reported by Obizoba & Atii [1991], who showed an improvement in lipid content in seeds germinated for 96 h, with a value of 5.7% compared to 3.1% for ungerminated sorghum seeds. Darwish et al. [2021] also found a 0.8% increase for seeds germinated for 72 h compared to ungerminated quinoa seeds.
Crude fiber content
The crude fiber content of sprouted seeds varied between 4.88 and 5.38 g/100 g DM (Table 1). Compared to the control (4.49 g/100 g DM), a considerable increase (p≤0.05) was observed in the seeds sprouted for 48–120 h. However, it should be noted that the crude fiber content did not differ significantly (p>0.05) between seeds germinated for 48, 72, 96 and 120 h. This significant increase compared to the control could be explained by the abundance of fiber in the early stages of germination [Guardianelli et al., 2022]. Pilco-Quesada et al. [2020] justified this change in fiber content by the loss of other nutritional components. This augmentation is consistent with the findings of Darwish et al. [2021], who highlighted an increase in the crude fiber of 1.77 g/100 g in C. quinoa seeds germinated for 72 h compared to the ungerminated seeds. Furthermore, Thakur et al. [2021] found that crude fiber content increased significantly with germination time.
Carbohydrate content
The carbohydrate content of sprouted seeds varied between 57.12 and 68.46 g/100 g DM (Table 1). Compared to the control (67.23 g/100 g DM), a remarkable increase (p≤0.05) was observed in the seeds sprouted for 24 h. Nevertheless, a noticeably lower (p≤0.05) carbohydrate content was noted in the seeds sprouted for 48 h to 120 h. The maximum reduction compared to the control was 9.43–10.11 g/100 g DM in the seeds germinated for 72–96 h. This substantial decrease could be attributed to the decomposition of complex carbohydrates into simple carbohydrates by enzymes activated during germination [Nelson et al., 2013] to provide the energy required for new plants [Ferreira et al., 2009]. Guardianelli et al. [2022] explained this reduction by the structure of starch, which facilitates its hydrolysis by endogenous amylolytic enzymes. Likewise, Elkhalifa & Bernhardt [2010] signaled that α-amylase was synthesized at low concentrations during the first hours of germination. As germination progressed, the concentration of α-amylase increased, breaking down starch into glucose.
Our results are in line with those reported by Pilco-Quesada et al. [2020], who observed a reduction of 8.1% in carbohydrates in the seeds germinated for 72 h compared with the control. In addition, Ramos-Pacheco et al. [2024] observed a non-significant decrease in carbohydrate content in seeds sprouted for 0 to 72 h. Guardianelli et al. [2022] reported that the content of starch, the main component of carbohydrates, decreased with germination time (≥24 h), especially in red C. quinoa.
To illustrate, the lower carbohydrate content observed in germinated quinoa seeds could be beneficial to health by helping to prevent diseases induced by high carbohydrate consumption, such as diabetes and cardiovascular disease.
Energy value
The energy value varied between 352.7 and 373.0 kcal/100 g DM (Table 1). The sprouted seeds tended to have lower energy value than the control, but a significant (p≤0.05) difference was found only between the seeds germinated for 72 h and ungerminated seeds with a decrease of 20.4 kcal/100 g DM. This decrease could be attributed to the low carbohydrate content. Our study results are consistent with those of Thakur et al. [2021], who found a 16.4 kcal/100 g reduction in quinoa seeds germinated for 72 h compared to the control.
Effect of germination time on ash content and mineral composition
The ash content fluctuated between 3.68 and 5.73 g/100 g DM with 3.87 g/100 g DM of the control seeds (Table 2). However, ash content of the ungerminated seeds did not differ significantly (p>0.05) from that of the seeds sprouted for 48 and 120 h. A noticeably higher (p≤0.05) ash content was determined in the seeds sprouted for 24, 72 and 96 h, with the highest increase compared to the control of 1.86 g/100 g DM noted in the seeds germinated for 24 h. This important enhancement was previously pointed out by Rao & Deosthale [1983] who reported that the high ash content in seeds germinated for 48 to 96 h may result from the treatment method used (reduction of humidity during germination), which concentrates the minerals. In contrast, the decrease in ash content may be due to the soaking and/ /or the transfer of minerals to the radicles during germination, where they act as co-enzymes in carbohydrate and protein catalysis [Bewley et al., 2013]. These radicles are then removed during the drying process.
Table 2
Ash content and mineral composition of quinoa seeds sprouted for different times.
The mineral composition of germinated quinoa seed varied with germination time. Manganese content varied between 9.41 and 21.24 mg/kg DM. Compared to the control, a marked decline (p≤0.05) was observed in the seeds germinated for 48, 96 and 120 h. The highest potassium content was found in ungerminated seeds (2,526 mg/kg DM) and it decreased significantly (p≤0.05) in the samples for subsequent germination times to a value of 438–559 mg/kg DM determined in the seeds germinated for 96–120 h. Calcium content ranged from 762 to 1,421 mg/kg DM. Relative to the control, a notable reduction of 482 mg/kg DM was observed in the seeds germinated for 48 h (p≤0.05). Regarding zinc, a significant difference (p≤0.05) was only found between the control (23.04 mg/kg DM) and the seeds sprouted for 96 h (16.33 mg/kg DM). No significant differences (p>0.05) were found between the seeds from different germination times with respect to iron and copper contents.
The important decline in the content of certain minerals could be explained by the germination method adopted and the use of distilled water, which allows the minerals to leach out [Bewley et al., 2013]. Furthermore, Kajla et al. [2017] explained that changes in mineral content during germination are due to the hydrolysis of organic complexes, releasing minerals that act as enzymatic co-factors and support macromolecules catalysis to provide the energy needed for germination. Our study results are close to those of Darwish et al. [2021], who observed a 39.43% enhancement in calcium content, in C. quinoa seeds. In addition, Bhinder et al. [2021] reported a decrease in manganese, zinc, and potassium with increasing germination time in quinoa seeds.
Effect of germination time on bioactive compound content
Total phenolic and total flavonoid contents
The total phenolic content of ungerminated and germinated quinoa seeds fluctuated between 83.5 and 133.8 mg GAE/100 g DM (Table 3). The highest value was found in the seeds germinated for 96 h, which was 50.3 mg GAE/100 g DM higher than that determined in the control. A marked augmentation (p≤0.05) was also observed in the seeds germinated for 48 and 72 h relative to the control. Regarding the total flavonoid content, the control seeds had the lowest content (25.6 mg QE/100 g DM). This value gradually increased with the extension of germination time up to 96 h and reached 99.1 mg QE/100 g DM. The total flavonoid content of the seeds germinated for 120 h was higher (p≤0.05) compared to the control, but did not differ significantly (p>0.05) from those of the seeds germinated for 24 and 48 h. The remarkable increases in total phenolic and total flavonoid contents could be attributed to the release of phenolic compounds from the cell walls [Alvarez-Jubete et al., 2010]. This increase is one of many metabolic changes that occur during seed germination, mainly due to the increased activity of the hydrolytic action of esterases and glucosidases on non-extractable phenolic compounds [Kim et al., 2016]. Kim et al. [2016] also noted that soaking seeds in water could activate enzymes, such as phenylalanine ammonia-lyase, which catalyzes the main phenylpropanoid reactions and, therefore, the formation of secondary metabolites.
Table 3
Total phenolic content, total flavonoid content, and saponin content of quinoa seeds sprouted for different times.
Our results concur with those found by Thakur et al. [2021], who observed a significant enhancement of 34.4% in the total phenolic content in quinoa seeds germinated for 72 h. A similar study by Alvarez-Jubete et al. [2010] determined an increase in the total phenolic content by 147.2% in quinoa seeds after 82 h of germination. In turn, Ramos-Pacheco et al. [2024] observed that contents of total phenolics and total flavonoids increased with increased germination time, especially in white C. quinoa. Therefore, germination can be an efficient means to improve the antioxidant properties of C. quinoa seeds.
It is important to note that the initial antioxidant composition of the seeds and their germination response can be influenced by the year of cultivation, the variety, genetic factors, and their interaction [Aguilar et al., 2019; Granado-Rodríguez et al., 2021a]. In addition, the region where the quinoa is grown and harvested, climatic conditions, the quality of the soil and the water used for irrigation, as well as farming practices determine the content of these compounds in the plant.
Saponin content
The saponin content of the ungerminated seeds was 1.06 g/100 g DM, and that of the germinated seeds varied between 0.48 and 1.42 g/100 g DM (Table 3). A significant (p≤0.05) decrease was observed compared to the control in the seeds germinated for 48 and 72 h.
Saponins are mainly found in the pericarp of C. quinoa seeds [Suárez-Estrella et al., 2020; Yadav et al., 2023], which contains about 86% of these secondary metabolites [Ruiz et al., 2017]. Considered as anti-nutrients [Granado-Rodríguez, et al., 2021b], saponins form insoluble complexes with certain minerals and vitamins, thereby reducing their intestinal absorption [Ruales & Nair, 1993; Zhou et al., 2023].
Saponin content in quinoa seeds depends on the variety [Granado-Rodríguez et al. 2021a; Mora-Ocación et al., 2022] and cultivation conditions such as rainfall, which can reduce saponin content [Lim et al., 2020]. The Titicaca, used in our study, is a bitter variety, with a saponin content above the 0.12% threshold set by the Codex Alimentarius [2019] as an acceptable limit to avoid bitterness. Therefore, the seeds of this variety require prior treatment before consumption, such as mechanical processing (polishing or sieving) and/or washing with water [Zhou et al., 2023]. However, mechanical treatments can negatively affect the nutritional profile of quinoa [Casalvara et al., 2024; Gómez-Caravaca et al., 2014], which facilitates treatment by washing or the use of alternative processing, such as germination, to remove saponins [Lan et al., 2024].
Our study results support those of Mhada et al. [2020], who found a saponin content of 2.03% in raw quinoa of the Titicaca variety. This level was reduced to 0.07% after polishing and processing into semolina. Furthermore, Nickel et al. [2016] reported a saponin content of 3.33% in raw quinoa, which became 2.75% after soaking for 15 min under a stream of running water. Similarly, Chaudhary et al. [2024] showed that soaking quinoa seeds in water for 24 and 48 h reduced the saponin content by 0.4% and 0.7%, respectively, compared to 1.9% saponin in raw quinoa. These reductions could be explained by the solubility of saponins in water [Bhinder et al., 2021]. Regarding germination, Suárez-Estrella et al. [2021] observed a reduction in saponin content from 0.40% to 0.05% in seeds germinated for 72 h. Similarly, Bhinder et al. [2021] reported that germination reduced saponin content in white and black quinoa after 96 h. Moreover, Darwish et al. [2021] found a 60% reduction in saponins in quinoa seeds germinated for 72 h.
As a result, it can be concluded that germination, particularly for 48 and 72 h, was an effective technique for partially reducing the saponin content of quinoa seeds, with a reduction of 0.58 g/100 g DM, i.e., a reduction of 54.72%. However, this technique is still not sufficient to completely remove the bitterness. According to Koziol [1991], bitterness is perceived by humans at saponin levels above 0.11%, which necessitates the use of additional techniques to reduce this bitterness, such as prolonged soaking, high-pressure washing, mechanical polishing and boiling of the seeds, to obtain a less bitter powder suitable for various food preparations.
Tocopherol content
The highest α-tocopherol content was determined in oil from the seeds sprouted for 24 h (6.45 µg/g oil) (Table 4). It was significantly (p≤0.05) increased by 1.63 µg/g oil compared to the control. Germination beyond 24 h notably (p≤0.05) reduced α-tocopherol content to 3.24–4.65 µg/g oil. In turn, the β-tocopherol content fluctuated between 1.14 and 4.14 µg/g oil, with noticeable differences (p≤0.05) observed in the seeds germinated for 48, 72, 96, and 120 h relative to the control. However, an increase (by 1.21 µg/g oil) was found only in the oil from seeds sprouted for 72 h. Samples analyzed at 48, 96 and 120 h had lower β-tocopherol content than the control. γ-Tocopherol content varied between 0.79 and 1.43 µg/g oil. Oil from the ungerminated seeds had significantly (p≤0.05) higher γ-tocopherol content than the oil from seeds sprouted for 72 h. Differences between the remaining samples were insignificant (p>0.05). Concerning δ-tocopherol, its content ranged from 1.35 to 4.05 µg/g oil. Compared to the control, an important increase was detected in the seeds germinated for 24, 48, 96, and 120 h (p≤0.05). with the highest augmentation of 2.67 µg/g oil found in the seeds germinated for 120 h.
Table 4
Tocopherol content of oil from quinoa seeds sprouted for different times.
α-Tocopherol, which is the most active form of vitamin E [Žilić et al., 2014], was the predominant tocopherol throughout the germination period in our study. This finding is consistent with those reported by Pachari Vera et al. [2019], who showed that yellow quinoa varieties had higher α-tocopherol levels compared to other forms. In addition, Tarasevičienė et al. [2019] reported an increase in α-tocopherol and a decrease in γ-tocopherol contents during germination in edible seeds such as wheat, radish, sunflower, lentil, and amaranth germinated for 24, 72 and 120 h. In turn, Granda et al. [2018] demonstrated that the quinoa varieties Amarilla de Marangani and Titicaca had high levels of α-tocopherol and low levels of γ-tocopherol, while δ-tocopherol content of 3.72 mg/kg and 4.59 mg/kg was found in the quinoa varieties Blanca Dulce and Black Quinoa, respectively. However, these results do not agree with those of Žilić et al. [2014], who reported γ-tocopherol as the most abundant tocopherol in quinoa seeds, followed by α-tocopherol, with small amounts of β- and δ-tocopherols, as also reported by Carciochi et al. [2016]. These dissimilarities could have been due to varietal differences [Tang et al., 2016], seed color [Granda et al., 2018], germination and extraction methods, as well as climatic and edaphic growing conditions.
Tocopherols play an important role during germination by scavenging free radicals and preventing lipid peroxidation to protect young seedlings from oxidative stress [Yang et al., 2018]. In addition, they have been suggested to elicit many health benefits. In particular, γ-tocopherol inhibits inflammation and the proliferation of prostate and colon cancer [Balakrishnan & Schneider, 2023]. Furthermore, Devaraj et al. [2008] highlighted the combined ameliorative effect of α- and γ-tocopherol on oxidative stress in patients with metabolic syndrome. These two tocopherols can readily quench free radicals [Balakrishnan & Schneider, 2023]. Likewise, tocopherols have been linked to the prevention of chronic diseases, disorders, and certain types of cancer [Ryynänen et al., 2004]. They are also known to regulate gene expression, signal transduction, and cellular functions [Shahidi & De Camargo, 2016]. In addition, Tarasevičienė et al. [2019] reported their role in reducing the production of thromboxane, a cytokine with vasoconstrictive effects, which could affect cardiovascular health. Finally, Rizvi’s review [2014] supported and described the various benefits of tocopherols, such as antioxidant protection, prevention of atherosclerosis, and reduction of prostaglandin levels.
CONCLUSIONS
Germination of quinoa seeds resulted in a remarkable change in the compositions of macronutrients, minerals, and bioactive compounds. Significant improvements in the contents of protein, crude fiber, total phenolics, and total flavonoids were observed in the seeds germinated for 96 h, indicating that prolonged germination was effective. A substantial decrease in carbohydrates was observed from 72–96 h of germination. The seeds germinated for 24 h had high levels of α-tocopherol and ash, while saponin content was reduced after 48 and 72 h of germination. In addition, the energy value of the seeds was reduced in those germinated for 72 h.
In this respect, the powder obtained from germinated quinoa seeds, especially for 96 h, has the potential to be used as a valuable ingredient in various food products e.g. in the production of savory snacks, offering new and innovative opportunities. In addition, it will be essential to raise consumer awareness of the benefits of powders obtained from germinated quinoa seeds. However, the development of additional processes to further reduce saponin levels is necessary to ensure their safe use and positive impact on public health.
These results are very promising for selecting quinoa varieties with the best nutritional properties to be grown in the semi-arid climate of eastern Morocco and for new cultivation environments.