INTRODUCTION
Bread is a staple source of carbohydrates commonly consumed worldwide, irrespectively of the country development [Conte et al., 2016]. The basic bread recipe consists of wheat flour, water, salt, and a leavening agent. The presence of gluten proteins in wheat (gliadin), barley (hordeins), and rye (secalins) guarantees the typical viscoelastic properties of bread. However, the same proteins can be harmful to a growing number of consumers who suffer from gluten-related disorders [Scherf et al., 2016]. In those individuals, the consumption of regular wheat bread can result in health complications and aggravation of the disease symptoms. Therefore, the demand for gluten-free (GF) alternatives has been increasing in the last few years. Gluten elimination from bread has a detrimental effect on its viscoelastic, technological, nutritional, and sensory properties [Conte et al., 2019]. The available GF bread alternatives are mainly produced with corn, rice, and other GF cereals having a lower content of vitamins, minerals, and proteins than wheat flour [Aguiar et al., 2021]. Importantly, these components also have a negative effect on the sensory properties of GF bread, including its aroma [Pico et al., 2017a]. To date, many efforts have been undertaken to improve the nutritional value, structure, and aroma of GF bread. Among the most studied additives are hydrocolloids, proteins, lipids and emulsifiers, which were found to improve the technological and viscoelastic properties of dough [Conte et al., 2020]. Moreover, the increasing popularity of fruit and vegetable by-products, additives rich in protein, fiber, and bioactive compounds, has been observed [Föste et al., 2020]. However, additives can significantly influence the aroma of GF bread, which is of particular importance since flavor is the key to market success [Heenan et al., 2008]. The pleasant aroma of traditional wheat bread is a result of fermentation, lipid oxidation, and Maillard reactions [Pico et al., 2017a]. The Maillard reactions, which occur between amino acids and reducing sugars, are responsible for the browning of crust and the distinctive roasted and nutty aroma [Cerny, 2008]. The components of GF products, such as proteins, lipids as well as sugars, act as precursors and strongly affect the generation of volatile compounds [Pico et al., 2017a]. However, because of the lower content of several amino acids, the aroma of GF bread is significantly different from that of wheat bread [Pico et al., 2017a].
Edible insects (EIs) are considered a source of proteins, lipids, fiber, and minerals of a high nutritional value [Montowska et al., 2019; Nissen et al., 2020]. Previous reports have shown that the incorporation of EIs can significantly improve the nutritional value of GF food products [Kowalczewski et al., 2021]. The Food and Agriculture Organization of the United Nations (FAO) emphasizes that EIs are highly nutritious and environmentally friendly due to their breeding conditions [FAO, 2013]. Regulation 2283/2015 on Novel Foods and its implementing Regulations 2468/2017 and 2469/2017 clarify the rules concerning edible insect’s application in food in the European Union (EU) [UE, 2015]. According to the European Food Safety Authority, mealworm larvae can be used as a whole, dried as snacks, and powdered as an additive in other various food products [Turck et al., 2021b]. Moreover, frozen and dried formulations from house crickets (Acheta domesticus) [Turck et al., 2021a] and partially defatted house cricket powder are approved as a novel food [Turck et al., 2022].
Edible insects likely to be accepted in Europe include mealworm and buffalo worm larvae as well as grasshoppers, crickets, and locusts [Skotnicka et al., 2021]. However, the European population is reluctant to consume whole EIs, in both larval and adult forms [Mishyna et al., 2020]. Recent studies have indicated that the growing consumer awareness of the environmental costs of meat production may improve the acceptance of alternative protein sources, including EIs [Ribeiro et al., 2022]. Crickets are one of the most important among the many described EIs. The A. domesticus is one of the most produced species of crickets, owing to the ease of breeding and good flavor characteristics of the cricket powder (CP), known also as cricket flour, obtained from it. Published studies have demonstrated a high nutritional value of proteins and lipids obtained from crickets [Psarianos et al., 2022]. Moreover, the biological activity of crickets has been widely described in the context of positive effects on human health. The most frequently reported are antioxidant and anti-inflammatory activities [Bernal, 2021], but the consumption of crickets has also been shown to improve the condition of intestinal microbiota [Stull et al., 2018] or reduce insulin resistance [Escobar-Ortiz et al., 2022].
CP-enriched GF bread is characterized by a unique bouquet of volatile organic compounds (VOCs) and its aroma resembles that of conventional bread [Nissen et al., 2020]. Hence, it seems worth investigating how the addition of protein-rich insect flour affects flavor generation in GF bread. Therefore, the aim of this study was to analyze the changes of VOCs in the experimental GF bread fortified with different contents of CP halfway through the baking time (15 min) and after baking (30 min). The analyses were performed both for the crumb and the crust of the CP-enriched breads. The sensory properties and the content of precursors of several VOCs, namely free amino acids (FAA), were analyzed as well.
MATERIALS AND METHODS
Materials
Rice starch was purchased from BENEO GmbH (Mannheim, Germany), potato starch from PPZ Trzemeszno sp. z o.o. (Trzemeszno, Poland), guar gum and pectin from Agnex (Bialystok, Poland), lyophilized yeast from Bakalland S.A. (Warsaw, Poland), sugar from Pfeifer & Langen Polska S.A. (Sroda Wielkopolska, Poland), salt from Ciech Soda Polska S.A. (Janikowo, Poland), rapeseed oil from ZT ‘Kruszwica’ S.A. (Kruszwica, Poland), and cricket powder from Crunchy Critters (Derby, United Kingdom).
Preparation of gluten-free bread
The control GF bread, marked as CP0, was made of the following ingredients: 200 g rice starch, 50 g potato starch, 4.25 g guar gum, 4.25 g pectin, 15 g yeast, 5 g sugar, 4.25 g salt, 7.5 g rapeseed oil, and 275 g distilled water. Firstly, both types of starch were mixed together, then, in the test samples, starch mix was replaced with CP in three different quantities of 2, 6, and 10% (w/w), and breads containing cricket powder were denoted as CP2, CP6, and CP10, respectively. The amounts of other components were unchanged. All the ingredients, except rapeseed oil, were combined and mixed for 2 min at a speed of 70 rpm using the KitchenAid mixer (model 5KPM5EWH, KitchenAid, Benton Harbor, MI, USA), then the oil was added, and mixing was continued for the next 6 min. The dough was fermented in a fermentation chamber for 20 min (temperature 35°C, relative humidity 85%) and punched. Each sample of dough was divided into two parts (230 g each) and placed in baking forms. The final fermentation was carried out for 15 min at 35°C. The prepared dough was baked at 230°C for 30 min, then the bread was left at room temperature for 3 h to cool down and sliced (about 1.5 cm thick). The analyzed breads were baked twice, and three samples were taken from each baking. The samples were taken within 15 and 30 min of baking time. Afterwards, the crust and crumb were carefully separated and ground for later analysis.
Free amino acid analysis
The profile of individual FAAs was analyzed as described previously [Drabinska, 2022]. Briefly, 0.5 g of freeze-dried samples were extracted with 3.5 mL of 50% (v/v) methanol (ISO reagent purity, Supelco, Bellefonte, PA, USA) for 20 min at 50°C using a laboratory shaker S50 (CAT Germany GmbH, Lehrte, Germany) at a speed of 800 rpm/min. Extracts were centrifuged at 5,500×g for 15 min (Universal 320 R cooled centrifuge, Hettich Holding GmbH & Co. oHG, Kirchlengern, Germany), and supernatants were collected in 10 mL volumetric flasks. The extraction was performed three times.
The extracts were directly analyzed using the EZ: Faast™ kit for free (physiological) amino acids (Phenomenex, Aschaffenburg, Germany) according to the manufacturer’s recommendations. The analytical procedure involves solid-phase extraction of 100 μL of the extract, followed by derivatization and liquid-liquid extraction. FAAs were separated using the ZB-AAA EZ Faast™ capillary column (10 m × 0.25 mm, Phenomenex) installed in an Agilent 7890A gas chromatograph (Agilent Technologies, Santa Clara, CA, USA) equipped with an autosampler G45134 and an Agilent 5975C mass selective detector. Helium was used as a carrier gas with a flow rate of 1.5 mL/min. The samples (2 µL) were injected in the split mode at a 1:15 ratio. The initial oven temperature was 110°C, then it was increased to 320°C (30°C/min). The injector and ion source temperatures were 250 and 240°C, respectively. Amino acids were identified and quantified using standards for each amino acid, and normalized with reference to the internal standard (norvaline).
Analysis of volatile compounds in bread samples
To extract VOCs from the headspace (HS) of bread (crumb and crust), carboxen/divinylbenzene/polydimethylsiloxane (CAR/DVB/PDMS) fiber of a 2 cm length was used (Supelco, Bellefonte, PA, USA). A 1.5-g portion of each sample was placed in a 20-mL glass vial. The sample was preheated to 50°C for 5 min in a heating block. This was followed by a 30 min extraction at 50°C. The solid phase microextraction (SPME) fiber was desorbed in the gas chromatograph injection port at 250°C for 10 min.
Comprehensive two-dimensional gas chromatography coupled with time of flight mass spectrometry (GC×GC-ToFMS) analysis of the VOCs was performed using an Agilent Technologies 6890N gas chromatograph (Agilent Technologies, Palo Alto, CA, USA) with a ZOEX cryogenic modulator (N2) coupled to a PEGASUS 4 time-of-flight mass spectrometer (LECO, St. Joseph, MI, USA). An Agilent Technologies GC sampler 80 was used to inject the samples. The compounds were separated on a nonpolar SLB-5 column (30 m × 250 µm × 0.250 µm; Supelco) as the firstdimension column, and a polar Supelcowax-10™ column (0.75 m × 200 µm × 0.1 µm; Supelco) as the second-dimension column. The oven program in the first dimension was as follows: initial temperature 40°C (1 min), 6°C/min to 200°C (0 min) and 25°C/min to 235°C/min (5 min). The oven program in the second dimension was: initial temperature 55°C (1 min), 6°C/min to 215°C (0 min) and 25°C/min to 250°C (5 min). Helium was used as the carrier gas at a flow rate of 0.8 mL/min. The modulation time was set to 4 s. The analyses were performed in the splitless mode. The temperature of the GC/MS transfer line was 260°C. Full spectral information (m/z range of 33–333) was acquired at a detector voltage of 1700 V at 150 spectra/s. The chromatograms were processed using LECO ChromaTOF® v.4.40 software, then the NIST 2.0 library was used for compound identification. The following parameters were used for the analyte match criteria: minimum similarity – 700, mass threshold – 10, and signal to noise ratio – 1000. Additionally, retention indexes were calculated and compared with literature data.
Sensory analysis
The aroma of the experimental GF products was evaluated by the quantitative descriptive analysis (QDA) method according to the International Organization for Standardization (ISO) 13299:2016 standard [ISO, 2016]. The evaluation was performed by 6 trained panelists. The samples were prepared by mixing crumbs with crusts in a 1:1 (w/w) ratio. Only the fully baked bread samples were analyzed. The odor attributes, determined in preliminary sessions, were: sour, roasty, cooked potato, caramel/chocolate, fatty, rice-like, floral, and earthy. The intensity of all attributes was ranked using a scale from 0 (not perceivable) to 3 (strongly perceivable).
Statistical analysis
For every test, three independent repeated measurements were made, unless stated otherwise. Results were analyzed using one-way analysis of variance independently for each dependent variable. The post-hoc Fisher’s least significant difference (LSD) test was used to identify statistically homogeneous subsets at α=0.05. Statistical analysis of the data was performed with the STATISTICA version 13.3 software (TIBCO Software Inc., Palo Alto, CA, USA). Moreover, to identify the differences in the VOCs distribution between the experimental GF breads, principal component analysis (PCA) was performed using SIMCA software package version 16 (Umetrics, Umea, Sweden).
RESULTS AND DISCUSSION
Free amino acids as precursors of volatiles
The contents of FAAs determined in the crumb and crust of the analyzed GF breads are presented in Table 1 and Table 2, respectively. Firstly, crust and crumb may be compared to determine any possible differences in the amino acids content between these parts. As shown in Table 1 and Table 2, nine essential amino acids (EAAs) were present in both the analyzed parts of the breads in a similar content range. Nevertheless, a minor lower content was determined in the crust part compared to the crumb for the majority of the EAAs. Tryptophan content was only notably lower in the crust than in the crumb. In terms of the composition of non-essential amino acids (NEAAs), no significant reduction was observed in the crust compared to the crumb. In fact, in some cases, the content of NEAAs was even larger in the crust of the bread.
Table 1
Amino acid composition in crumbs of gluten-free bread without cricket powder (CP0) and with 2, 6, and 10% of cricket powder, presented as CP2, CP6, and CP10, respectively (µmol/g dry weight).
Table 2
Amino acid composition in crust of gluten-free bread without cricket powder (CP0) and with 2, 6, and 10% of cricket powder, presented as CP2, CP6, and CP10, respectively (µmol/g dry weight).
The influence of baking time (15 vs. 30 min) on the amino acid composition of bread crumbs and crusts (Table 1 and Table 2) was also investigated. The prolonged baking time did not affect the total content of both EAAs and NEAAs of breads fortified with CP. The significant (p<0.05) differences in the total content of EAAs and NEAAs were noted for CP0 in crust as well as CP0 and CP2 in crumb. For individual FAAs, most of the changes in their content after 15 vs. 30 min of baking were minor and for the majority of them statistically not significant. Therefore, it was concluded that baking time did not affect the content of FAAs in crumb and crust of the analyzed breads.
The last studied factor was the amount of CP additive. Four different types of bread (without CP, with 2%, 6%, and 10% of CP), separately for crusts and crumbs, were compared (Table 1 and Table 2). Not all changes between CP0 and CP2 were statistically significant. In the crust of breads baked for 15 min, significant (p<0.05) changes were observed in the content of two EAAs: lysine and methionine; and three NEAAs: proline, asparagine, and aspartic acid, while in the crumb of breads baked for 15 min only the content of threonine, alanine, glycine, proline, and glutamic acid changed significantly. Insignificant differences (p≥0.05) were noted for the total contents of both EAAs and NEAAs in crusts of CP0 and CP2 baked for 15 min, while in the crumb the statistically significant difference (p<0.05) was observed only for total NEAAs content. Significant differences were also noted between crusts of CP0 and CP2 baked for 30 min in respect of total content of EAAs and NEAAs. Interestingly, these differences were not always reflected for individual FAAs; a significant (p<0.05) difference was found for tryptophan (EAA), and five NEAAs: alanine, glycine, proline, aspartic acid, glutamic acid, and tyrosine in GF bread crust. A similar relationship was observed for isoleucine, lysine, alanine, glycine, proline, asparagine, aspartic acid, and tyrosine in GF bread crumb. In comparison to CP0, the addition of 6% of cricket powder resulted in significant differences in the content of the majority of FAAs, both in the crust and the crumb of model bread. In the CP10 bread, the content increased notably for all analytes. In the outer and the center of the bread baked for 15 min, the total content of EAAs doubled (CP0 vs. CP10), while the content of NEAAs increased 2.3 times. In the samples baked for 30 min, these changes were even larger; the total content of EAAs in CP10 crust and crumb was 2.6 times higher than in CP0, and the total NEAAs increased 4 times.
It is common knowledge that deficiencies of protein and EAAs, such as tryptophan, are common in individuals affected by gluten-related disorders. They may contribute to lower serotonin production and result in psychological symptoms observed in patients with celiac disease [Drabinska et al., 2018]. These deficiencies are due to a coupled effect of disturbed intestinal absorption and lower protein quality of GF products. Therefore, the increment of both FAAs and protein content is preferable in the products dedicated to people with gluten-related disorders. It should be mentioned that CP was characterized by a high protein content of approx. 60%, which is significantly higher compared to different types of flour [Kowalski et al., 2022] and its use in GF bread preparation significantly increased the total protein content of the final product [Kowalczewski et al., 2021]. Moreover, FAAs are precursors of volatile compounds which might modify the aroma profile. As a consequence, their impact on the flavor is inevitable, which is discussed in the further parts of the manuscript.
Profile of VOCs in GF bread
The analysis of VOCs in the CP-enriched bread provided important information regarding its flavor quality. A wide range of VOCs can be formed depending on the available amino acids and the reaction conditions [Schwab et al., 2008]. Among them are the products of Maillard reactions which occur at elevated temperatures and are especially important in breadmaking.
Comparing the composition of volatiles in the crust and crumb of analyzed breads multiple differences were observed (Figure 1). Moreover, the time of baking influenced the profile of the analyzed volatile compounds. In the crumb of breads baked for 15 min, the dominant group of volatiles was alcohols. After a longer time of baking, the proportions changed in all the samples of crumbs. The abundance of alcohols was found to be lower in both crumbs and crusts of the samples treated with high temperature for a longer time. This was accompanied by an increase in the abundance of pyrazines and sulfur compounds. With prolonged baking, the content of ketones in the crumb increased, while it decreased in the crust. In both the crumb and crust, 30 min of baking resulted in a lower percentage of esters.
Figure 1
Percentage content of acids, alcohols, aldehydes, esters, furans, ketones, terpenes, pyrazines, and sulfur compounds in crumb and crust of breads supplemented with 2, 6, and 10% of cricket powder (CP) and baked for 15 and 30 min.
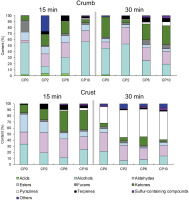
In the crust part of breads, the composition of volatiles after 15 min of baking was different than in crumbs. The abundance of alcohols was notably lower in crust than crumb, while aldehydes and esters were definitely more abundant in the crust. Major changes occurred in the crumbs of breads baked for 30 min, since longer baking time resulted in multiple Maillard reaction products. The most important observation, which differentiates the crust from crumb, is related to the content of pyrazines, the abundance of which was relatively low in the crumb.
Detailed results of the VOCs analysis are given in Table S1 (for crumbs) and Table S2 (for crusts). A total of 96 VOCs were identified in all the analyzed samples. These included: 3 acids, 16 alcohols, 13 aldehydes, 11 esters, 6 furans, 10 ketones, 18 pyrazines, 6 terpenes, 5 sulfur compounds, and 8 other components. Tables S1 and S2 also include the areas of peaks obtained in the GC×GC-ToF-MS analysis. A tentative identification was performed using GC×GC-ToF-MS data, and only the compounds with high scores of mass spectra (>90%) were presented. Additionally, confirmation of the identity was performed by comparing the obtained retention indexes with literature data. Acids were detected in the crumb of CP0, CP2, and CP6, while they were not present in the CP10. Acetic acid is most likely responsible for the sour aroma of rye bread since it was listed as one of its key odorants [Boeswetter et al., 2019]. Because of the polar character of acids, they show poor yields when extracted by SPME from the HS. Acetic acid (1) was not visible in the chromatograms of the CP10 samples. This might have been a result of its relatively low content since part of rice flour was replaced with CP. Another possible reason might have been competition phenomena [Wieczorek et al., 2022], the result of increased content of non-polar VOCs present in the HS above the bread containing a larger amount of CP. Moreover, it was reported previously that acetic acid was one of the compounds whose content changed significantly depending on the yeast culture used in the process [Hansen & Hansen, 1996]. Although the yeast culture was not changed in this study, the modification of matrix composition caused by the partial replacement of starch by CP could have affected the metabolism of yeasts. It is yet another likely explanation for the lower acid content in the GF bread containing larger amounts of CP. The effect of CP on yeast fermentation has never been analyzed; therefore, it is only a speculation, which is, however, worth elucidation in the future. Additionally, the presence of acids was only detected in the crumbs of GF breads, while no peaks corresponding to acids were observed in their crust. This was probably the effect of their evaporation from the outer part of the bread.
Food fermentation by yeast is accompanied by the formation of aliphatic and aromatic alcohols, known as fusel alcohols [Hazelwood et al., 2008]. When present in high contents, fusel alcohols cause the occurrence of off-flavor in food. However, low contents of these compounds in a product might result in a pleasant aroma. In the analyzed GF breads, the abundance of the majority of alcohols was found to decrease with a prolonged time of baking (Table S1 and S2). Moreover, larger peak areas for many of the individual alcohols were observed in the crumbs than in the crusts, especially for 1-hexanol (8). This can be explained by the evaporation of alcohols from the outer surface during heating. The addition of CP to the GF bread did not increase the content of alcohols. Moreover, their peak areas were lower in the breads with CP. A more detailed analysis reveals that 1-hexanol (8) was the predominant alcohol in the crumb of CP0 (15 min of baking) and CP10 (30 min of baking), while 1-pentanol (13) was the most abundant one in CP10 bread baked for 15 min. While in the crust part, the content of 1-hexanol (8) was lower than the contents of phenylethenyl alcohol (15), 1-propanol (16), and 2-methyl-1-propanol (17) in CP0. Some bias in the results was observed, probably because of the applied extraction technique. As presented in Table S1, the level of phenethyl alcohol (15) decreased with an increasing percentage of CP. Phenylethyl alcohol is a fusel alcohol that is produced from phenylalanine in the Ehrlich pathway during yeast fermentation [Pico et al., 2017b]. The content of phenylalanine increased with greater CP addition (Table 1), therefore an increase of phenylethyl alcohol could be expected. Thus, there is no reasonable explanation for its lower abundance in the CP-enriched breads. The observed lower content of alcohols, including the latter, may be explained by the competitive nature of SPME extraction. This point should be further investigated, since it is a common issue in food analysis [Spietelun et al., 2013].
Next important group of compounds affected by the incorporation of CP are aldehydes (Figure 1). They are secondary breakdown products of unsaturated lipids [Shahidi, 2001] as well as precursors of alcohols in the Ehrlich pathway, formed from acids [Hazelwood et al., 2008]. Their significance in the development of aroma of food products has been discussed recently [Dunkel et al., 2014]. Some of them, like hexanal, 2-nonenal or 2,4-decadienal, were recognized as the main odorants in most of food products [Dunkel et al., 2014]. Among the analyzed GF breads, the amount of hexanal (27) was found noticeably higher in the CP-enriched samples (Tables S1 and S2). Noteworthy, the content of this VOC was higher in the crust, compared to the crumb of the analyzed breads. The increment of its amount in the bread enriched with CP was probably a result of the high lipid content in CP. A previous study demonstrated lipid content of 29 g/100 g in CP [Montowska et al., 2019]. Oxidation of fatty acids is one of the ways in which aldehydes are formed in food. This process is accelerated by heating, which is a likely reason behind the increased abundance of aldehydes in the crust compared to the crumb of the analyzed GF breads.
In terms of peak area comparison between breads with different CP contents, the major differences were observed in the level of pyrazines in the crust part (Table S1 and S2). These compounds are formed in bread from the reaction of amino acids with sugars via the Maillard reactions. This process is well documented [Yu et al., 2021]. Pyrazines are known as important contributors to the pleasant aromas of bread; and many efforts have been made to promote their formation in GF bread [Pacynski et al., 2015]. Therefore, they gained special attention due to their probable influence on the bread’s flavor. Many pyrazines were formed in the crusts of breads with CP baking of 30 min, including: 2-ethylpyrazine (61), 3-ethyl-2-methylpyrazine (62), 2-ethyl-6-methylethylpyrazine (63), 2-isopropenylpyrazine (67), 5-ethyl-2,3-dimethylpyrazine (69), 2-methyl-3-(2-propenyl)pyrazine (70), 2-isobutyl-3-methylpyrazine (71), 3,5-diethyl-2-methylpyrazine (72), 2,3-diethylpyrazine (73), 2-ethyl-3,5-dimethylpyrazine (74), 2-acetyl-3-ethylpyrazine (75), 2,5-dimethyl-3-(2-methylpropyl) pyrazine (76), and 2-isoamyl-6-methylpyrazine (77) (Table S2). These compounds were not detected in the control bread – CP0. The increased content of pyrazines was determined in the breads with larger CP content, probably due to the higher content of FAAs. The abundance of pyrazines was higher in the crust than in the crumb, which can be explained by the greater exposure of the outer surface of bread to higher temperatures during baking. This intensified the Maillard reactions. However, some compounds, such as 2,3,5-trimethylpyrazine (68), were present in the crust part only after 15 min of baking; it was not detected after 30 min of baking. This suggests that this compound was further transformed or degraded at a quicker rate when exposure to heat was greater. Moreover, this compound was found in the crumb of the bread with its abundance increasing together with CP percentage (Table S1). A significant increase was also noted in the peak area of 2,5-dimethylpyrazine (65). Even though the affinity of this compound to the SPME fiber is low (its log P=0.6), it was present in high abundance, which indicates its high content. Therefore, despite its odor threshold being relatively high (800 ppb, according to Leffingwell & Associates [2018]) it might have been involved in the final aroma development. Its odor notes were described in the literature as chocolate, roasted, and earthy [Leffingwell & Associates, 2018]. Many of the pyrazines detected in this study (Tables S1 and S2) are characterized by low odor thresholds [Müller & Rappert, 2010]; therefore, their influence on the flavor seems inevitable.
The content of furans (described as furan and furan derivatives) was notably higher in bread without CP addition than in the ones containing CP (Figure 1). Furans are formed in the Maillard reactions [Srivastava et al., 2018], but the mechanism of their formation remains unclear. It has been proposed that there are multiple precursors and alternative routes for the formation of furans in foods rather than a single mechanism. The major routes are the thermal degradation of carbohydrates and/or ascorbic acid and its derivatives, and the thermal oxidation of polyunsaturated fatty acids [Crews & Castle, 2007]. The abundance of furans increased with the increasing percentage of CP in GF formulations (Tables S1 and S2), which, similarly to the case of pyrazines, can be explained by the intensification of the Maillard reactions that resulted from to the greater availability of FAAs. Similarly, to pyrazines, furans were found more abundant in the crust, i.e., the bread part that is more exposed to high temperatures. Some furan derivatives, such as 2-methylfuran (47), 2-ethylfuran (49), etc., were present at different levels, without any unambiguous dependence on the CP content. Since there is a lack of literature data regarding the possibility of their mutual transposition, it is not possible to present a likely explanation of these results.
Many VOCs presented in Tables S1 and S2 show aroma activity, some of them, detected only in CP-fortified bread, were reported as key odorants in wheat bread [Rohleder et al., 2019]. For instance, methional (88) was detected in the crumb of CP6and CP10, and in the crust part of all the breads with CP baked for 30 min. This is a further indication of the improvement of aroma quality of GF bread resulting from CP addition.
Due to the complexity of VOCs data, PCA was performed to reduce the dimensionality of the original data and the biplots are presented in Figure 2 separately for the crumb (A) and crust (B). For the crumb (Figure 2A), a good separation between the analyzed GF breads was observed. Breads CP0 and CP2 were positioned on the right side of the plot, while CP6 and CP10 – on the left side. CP0 was characterized by a higher abundance of the majority of alcohols and esters, while ketones, aldehydes, and sulfur compounds were associated with CP10. For crumbs, there was no differentiation between the baking times. For crusts (Figure 2B), there was no separation between the analyzed GF breads baked for 15 min, which all were placed in the bottom right quarter. In the case of the samples baked for 30 min, a clear separation of CP10 can be observed with its placement on the left side of the plot. Noteworthy, in accordance to the results described above, CP10 bread was characterized by the highest abundance of pyrazines and aldehydes.
Sensory analysis
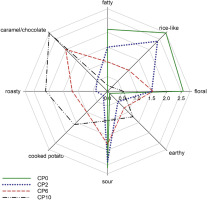
The results of sensory analysis of the aroma of fortified GF breads are presented in Figure 3. The reference bread, with no CP addition, was characterized mainly by “rice-like”, “sour”, “fatty” and “floral” notes. These aroma notes were perceived with lower intensity as the CP percentage increased. The addition of CP resulted in increasing intensity of “caramel/chocolate”, “roasty” and “cooked potato” notes. The GF breads enriched with CP were characterized by notes typical for wheat bread, such as nutty, caramel-like and roasted, that are associated with pyrazines. These results indicate that CP can be a valuable ingredient used for the manufacture of GF products with improved flavor characteristics, which currently are rather low [Alencar et al., 2021]. Numerous literature data indicate that bakery products are often enriched with powdered forms of various EIs, e.g., crickets, termites, grasshoppers, locusts, or mealworms [Yazici & Ozer, 2021]. The inclusion of edible insects into the recipe of these products affects not only the nutritional value but also the sensory properties of the end products. Zielinska et al. [2021] analyzed sensory acceptance of muffins enriched with different levels of cricket (Gryllodes sigillatus) and mealworm (Tenebrio molitor) powders. The results of their analysis showed that the EIs-enriched muffins were well-evaluated and fully accepted by consumers. Similar results were presented by Pauter et al. [2018], who also analyzed CP muffins. On the other hand, Smarzynski et al. [2021] showed that a small, 2% addition of CP to cookie formula improved the acceptance of final products.
CONCLUSIONS
The addition of CP influenced the sensory quality of GF breads. The nutritional quality in terms of the FAAs profile was also improved. This is of particular importance for the development of products dedicated to people on elimination diets. Content of both NEAAs and EAAs increased as a result of CP incorporation. Statistically significant changes were observed already at 4% CP addition. The higher content of FAAs contributed to the development of a richer aroma in the GF breads, which became similar to that of traditional wheat-based bread. This outcome is clearly related to the higher level of pyrazines in the CP-fortified GF breads, as the compounds of this group are responsible for the pleasant nutty and roasted notes. The analysis of VOCs in the experimental GF breads indicated that the addition of CP changed the composition of the volatile fraction in a significant way. Besides the case of pyrazines, major changes included increased aldehydes content, which was a likely result of the higher content of their precursors that were introduced with CP. The differences in the composition of volatiles affected the aroma of bread in a meaningful way which was confirmed by sensory assessment. Therefore, it can be concluded that CP is an attractive additive that shows potential for use in improving the sensory and nutritional properties of GF bread.