INTRODUCTION
Fish and seafoods are abundant sources of good protein, oil, and minerals for maintaining healthy body worldwide [Ravichandran et al., 2012]. While fish is being considered for various preparations, large amounts of fish by-products (head, viscera, skin, and bones) are generated (approximately 50–60% of total fish capture) during processing. In many countries, these fish by-products have not been utilized efficiently and their disposal as such can have large adverse impacts on the natural environment [Jafarpour et al., 2020]. Therefore, there is an urgent requirement to find ecologically-sustainable way for efficient utilization of fish by-products. The fish by-products are potential sources to obtain high-quality protein hydrolysates by using enzymatic hydrolysis [Opheim et al., 2015; Slizyte et al., 2018]. In our previous study we utilized fish head and viscera to produce protein hydrolysate using Alcalase which was then microencapsulated [Kumari et al., 2023].
In urban areas, people do not have enough time to cook foods due to their busy schedule, which results in the consumption of fast food having high sugar, fat, and salt contents, and being deficient in protein, fiber, vitamins, and minerals. Consumption of such types of foods causes malnourishment and nutrition-related diseases [Farzana et al., 2017]. The problem of malnutrition related to protein deficiency is a matter of great concern in both developing and developed countries. The daily allowance of protein recommended by National Institute of Nutrition (ICMR NIN) in India is 54 g/day for men, 46 g/day for women and 23 g/day for children [ICMR NIN, 2020]. Nutrient-enriched foods, such as protein-enriched ready-to-cook soup, are one of the potential ways to fight against malnutrition as they are easy to cook and eat [Mohamed et al., 2020]. The underutilized fish or fish by-products are potential sources for the development of protein-rich products which can help both high- and low-income populations to fight against malnutrition and, on the other hand, the fish industry can also earn extra profit by producing value-added products and aid in eliminating harmful effects on the natural environment.
Fish have been utilized to produce protein-rich products, such as fish soup powder, fish protein concentrate, etc. [Shashidhar et al., 2014]. However, the products developed from fish-derived ingredients may have a low market acceptability due to sensory problems (flavor and fishy odor) despite their improved nutritional quality. Therefore, in this study, microencapsulated protein hydrolysate obtained from pink perch head and viscera was used to formulate a ready-to-cook soup mix. The developed ready-to-cook soup mix may have improved sensory and nutritional properties, which increases the market acceptability of fish products. The optimization of pink perch head and viscera protein hydrolysis, characterization of hydrolysate, and its controlled release from microencapsulate were studied in our previous study [Kumari et al., 2023]. Therefore, the aim of this study was to formulate a ready-to-cook (RTC) soup mix containing the microencapsulated protein hydrolysate of pink perch head and viscera and evaluate its physical and chemical characteristics as well as its consumer acceptability.
MATERIALS AND METHODS
Raw material
The pink perch (Nemipterus japonicus) head and viscera were procured from Ulka Seafood Pvt. Ltd. (Mumbai, India) and minced into Hobart mincer (model AE 200, Hobart, Offenburg, Germany) with 10 mm holes. Vegetables (bean, carrot, and pea) used in soup formulation were procured from the local market (Noida, Uttar Pradesh, India). The vegetables were cleaned and cut into pieces. The chopped vegetables were blanched and freeze-dried in a lyophilizer (SNS FD-50, SN Solutions, Noida, Uttar Pradesh, India) and stored at −20°C till further used. The other ingredients, such as: salt, sugar, potato starch, milk solid, wheat refined flour, sunflower oil and spice mix (turmeric, black pepper, and roasted cumin), were purchased from the local supermarket. The sun-dried whole fish powder was procured from Coastal Export Corporation (Mangalore, Karnataka, India). All the chemicals and reagents used were of analytical grade. Nutrient agar, Czapek dox agar, XLD agar and EMB agar media were procured from Himedia (Thane, Maharashtra, India).
Preparation of microencapsulated protein hydrolysate
The protein hydrolysate was prepared as per method of Kumari et al. [2023]. Pink perch head and viscera minced sample (100 g) was mixed with hot distilled water (100 mL). Alcalase® (Novozymes A/S, Bagsværd, Denmark) (0.15 mL) was added into the sample. The content was incubated at 58°C for 85 min on a rotating test tube mixer (Stuart SB3, Vernon Hills, IL, USA) to ensure homogenous mixing. After completion of hydrolysis, the enzyme was deactivated by heating the mixture at 90°C for 10 min. The mixture was centrifuged at 5,000×g for 15 min. After the centrifugation, the tubes containing three layers were frozen at −20°C. The middle layer containing the protein hydrolysate was separated by cutting the layer with a knife. The protein hydrolysate layer thus obtained was freeze-dried in a lyophilizer (SNS FD-50, SN Solutions). The freeze-dried protein hydrolysate was stored at −20°C.
The freeze-dried protein hydrolysate was microencapsulated as per method of Kumari et al. [2023]. Maltodextrin (dextrin maize starch), gum Arabic and sodium alginate (1:1:1 ratio, w/w/w) were used as wall material. The protein hydrolysate and wall material were used in 4:6 ratio (w/w). First, the protein hydrolysate was dissolved in distilled water (1:25 ratio, w/v) with continuous stirring on a magnetic stirrer. Then, the wall material was added gradually in the following order: first maltodextrin then gum Arabic followed by sodium alginate with continuous stirring on a magnetic stirrer for 4 h. The microencapsulated protein hydrolysate was freeze-dried in a lyophilizer. The freeze-dried microencapsulated protein hydrolysate was stored at −20°C until further used.
Formulation of ready-to-cook soup mixes
The freeze-dried microencapsulated protein hydrolysate was used for RTC soup (microencapsulated protein hydrolysates soup, MPHS) mix formulation (Figure S1). Freeze-dried protein hydrolysate alone and sun-dried whole fish powder-based RTC soup (protein hydrolysate soup, PHS, and fish power soup, FPS, respectively) mixes were also prepared and used as controls. The composition of three RTC soup mixes and blank sample are provided in Table 1. The RTC blank soup (BS) mix was prepared by traditional standard recipes. The RTC soup mixes were prepared by mixing all the ingredients, i.e., vegetables, potato starch, wheat refined flour, sunflower oil, salt, sugar, and spices mix, and were packed in metallic polyethylene zip lock bags (32-micron thickness) with proper hygiene in a laminar flow. The packages were stored at −20°C. The RTC soup mix (each type) was prepared in triplicate. The main idea of formulation was to use different sources of proteins/peptides/amino acids and achieve 15% protein equivalent content in all RTC soup mixes. Therefore, the amount of other ingredients varied or was adjusted to make the overall weight to 100 g. The formulated RTC soup mixes were also compared with commercially available chicken soup (CCS) and vegetables soup (CVS). The ingredient used in CCS were refined wheat flour (maida), sugar, milk solids, hydrolyzed vegetable protein, iodized salt, flavors (nature identical flavoring substances and garlic), dehydrated vegetables (onion, leeks), dehydrated chicken, stabilizers (E461 and E407), antioxidant (E300), palm oil, thickener (E415), flavor enhancers (E627 and E631), spices and condiments (black pepper) and food color (caramel IV). The ingredients used in CVS were maize starch, dehydrated vegetables (carrot flakes, cabbage flakes, green peas, corn, onion powder, leeks), refined wheat flour (maida), milk solids, iodized salt, sugar, hydrolyzed vegetables protein, palm oil, thickener (E415), spices and condiments, anticaking agent (E551) and flavor enhancers (E627 and E631).
Figure 1
Viscosity at different shear rates (A) and shear stress at different shear rates (B) of ready-to-cook soup mixes. BS, blank soup; MPHS, microencapsulated protein hydrolysate soup; PHS, protein hydrolysate soup; FPS, sun dried whole fish powder soup; CCS, commercial chicken soup; CVS, commercial vegetable soup.
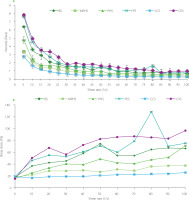
Table 1
Formulation of ready-to-cook soup mixes (g/100 g).
Characterization of ready-to-cook soup mix
Proximate analysis
The moisture, lipid and ash contents of ready-to-cook soup mixes were determined as per the standard methods of AOAC International [AOAC, 2005]. The moisture content of RTC soup mixes was determined by hot air oven method (Method no: 935.29). The RTC soup mix (1 g) was kept at 105°C for 24 h in a hot air oven. The sample was cooled in a desiccator and then weighed. Lipid content of the ready-to-cook soup mixes was determined using a Soxhlet apparatus (Method no: 2003.05). Ash content of the ready-to-cook soup mix was determined with the gravimetric method using a muffle furnace. RTC soup mixes (1 g) were kept at 500°C for 24 h in a muffle furnace. After complete ashing, weight of the ash was measured after sample cooling in a desiccator (Method No: 942.05).
The total nitrogen content of the ready-to-cook soup mix was estimated by Dumas method [Feng et al., 2022] in an automated micro-analyzer (Elementar® Max N Exceed, Elementar Analysensysteme GmbH, Langenselbold, Germany). The sample (50 mg) was weighed in a tin crucible and kept in the combustion chamber. The standard (aspartic acid) of known nitrogen content was used to calculate the amount of nitrogen present in the sample. The total protein content was calculated by multiplying the total nitrogen content by a factor of 6.25 [Steinsholm et al., 2021].
The carbohydrate content of the soup mix (g/100 g) was calculated using formula (1) [Merrill & Watt, 1973].
All the analyses were performed in duplicate.
Total soluble solid determination
Total soluble solid (TSS) content of the ready-to-cook soup mixes was measured using refractometer (CTL-REFM-BR32, LW Scientific, Lawrenceville, GA, USA) at room temperature. An aliquot of 3 g of the soup mix was dissolved into 40 mL of hot water and cooked for 5 min. A drop of sample was taken on a refractometer prism and observed through an eyepiece. The TSS of the sample was expressed in °Brix. The analysis was conducted in duplicate.
Bulk and tapped density determination
Bulk density (BD) and tapped density (TD) were determined following the method described by Fasogbon & Taiwo [2019]. The RTC soup mixes (5 g) were taken into a 10 mL empty graduated cylinder. The tapped density was determined after continuous tapping of the graduated cylinder (an average of 300 beats), as long as the volume of the dispersions of RTC soup mixes changed. The BD and TD were expressed as weight of sample per unit the volume occupied in the cylinder (g/mL). The flowability of RTC soup mixes was also determined by Hausner ratio (HR) and compressibility index (CI). The formulas (2) and (3) were used to calculate the HR and CI, respectively.
Solubility determination
The solubility of the RTC soup mixes was determined as per the method described in the paper of Cano-Chauca et al. [2005] with some modifications. The sample (1 g) was dissolved in 100 mL of distilled water with a continuous stirring on a magnetic stirrer for 4 min. The solution was centrifuged at 5,000×g for 4 min. The supernatant (25 mL) was dried in an oven at 105°C for 3–5 h. Weight of the dried solid matter as a percentage of the initial powder was used to determine sample solubility in water.
Viscosity determination
The viscosity of the RTC soup mixes was determined as per the method of Noordraven et al. [2021] using an MCR 52 rheometer (Anton Paar, Graz, Austria). The RTC soup mixes were prepared by dissolving 10 g of RTC soup mix into 120 mL of hot water and boiling for 5 min. The prepared soup was taken into a concentric cylinder cup and placed in a rheometer. The measurement was done using an SC4 21 spindle and at constant temperature (60°C). The measurement of viscosity (Pa×s) was performed by increasing the shear rate from 1 to 100 1/s. Results were expressed as the curves of viscosity vs. shear rate and shear stress (Pa) vs. shear rate. All the measurements were done in duplicate.
Pasting properties determination
Pasting properties, i.e., pasting temperature, peak time, peak viscosity, trough viscosity, final viscosity, breakdown (peak viscosity minus trough viscosity), and setback (final viscosity minus trough viscosity) were determined as per the method of Hanan et al. [2020] using a rheometer (MCR 52, Anton Paar). The 1 g sample was weighed in a starch pasting cell (ST 24-2D) and dissolved in 40 mL of distilled water. The soup dispersion was equilibrated at 50°C for 1 min and heated up to 95°C at the rate of 0.2°C/s rate. The soup dispersion was kept at 95°C for 2.5 min and then cooled to 50°C at the rate of 12°C/min rate. The dispersion was again held for 2 min at 50°C. The paddle rotated at 960 rpm speed for the first 10 s to mix the sample and then at a constant speed of 160 rpm. All the parameters were expressed in mPa×s except pasting temperature which was in °C and peak time which was in min.
Consumer assessment of the ready-to-cook soup mixes
The consumer test of the ready-to-cook soup mixes was performed using a 9-point hedonic scale [Watts et al., 1989]. The assessment was conducted in the semi-scale with 30 semi-trained assessors (15 men, 15 women, age range 24–45) recruited from students and employees of the Amity University Uttar Pradesh, Noida, India. The consumer assessment of RTC soup mixes was done after dissolving 10 g of RTC soup mix into 120 mL of hot water and boiling for 5 min in an open vessel. Unlabeled warm soup was served to each assessor in a paper cup. The assessors were asked to clean their palates with water before tasting the second sample. The 9-point hedonic scale was provided to rate the sample with 9 as liked extremely, 8 as like very much, 7 as like moderately, 6 as like slightly, 5 as neither like nor dislike, 4 as dislike slightly, 3 as dislike moderately, 2 as dislike very much, and 1 as dislike extremely. The assessors were asked to give their remarks about appearance, taste, odor, flavor and overall acceptability of each of the sample [Wang et al., 2010]. The overall acceptability means acceptability of the products by assessors in total as a combination of all sensory characteristics.
Determination of storage stability of the ready-to-cook soup mixes
The accelerated shelf-life testing (ASLT) was used to accelerate the rate of product deterioration without altering the mechanisms of change when stored at room temperature. ASLT was used to predict the shelf-life of products in short time. The storage study of the RTC soup mixes packed in metallized polyester zip lock bag (32 micron) was done at 40°C and 75% relative humidity (RH) for 15 days. The experiment was set-up in laminar air flow. The samples were drawn at regular intervals of 3 days [Bhatt et al., 2020] and subjected to analysis of lipid quality, pH, color parameters, and microbial count.
2-Thiobarbituric acid reactive substance estimation
2-Thiobarbituric acid reactive substance (TBARS) contents of the ready-to-cook soup mixes were determined as per the method of Schmedes & Holmer [1989]. The 5 g sample was dissolved in 25 mL of 20% (w/v) trichloroacetic acid solution. The contents were mixed thoroughly. The suspension was filtered to remove solid particles. An aliquot of 2 mL of the aqueous 2-thiobarbituric acid solution (0.02 M) was added to 2 mL of the filtrate in a test tube. The tube was incubated at 100°C for 30 min and then cooled under running tap water. Absorbance was measured at 532 nm using a UV-Vis spectrophotometer (LMSPUV1900S, Labman, Chennai, India). TBARS value was calculated from a malondialdehyde standard curve and expressed as mg malondialdehyde per kg of the ready-to-cook soup mix.
Peroxide value determination
The peroxide value (PV) of ready-to-cook soup mix was determined as per the AOAC method no: 965.33 [AOAC, 1999]. The portion of 3 g of ready-to-cook soup mix was weighed in a conical flask, and 30 mL of acetic acid-chloroform solution (3:2, v/v) was added to dissolve the lipids. The suspension was filtered through Whatman no. 1 filter paper to remove the solid particles. A saturated potassium iodide solution (0.5 mL) was added to the filtrate. A few drops of a starch solution were added to the filtrate and titrated with sodium thiosulfate (0.01 N). The PV was calculated using the formula (4) and expressed as milli equivalent peroxide per kg of ready-to-cook soup mix.
where: S, volume of sodium thiosulphate used (mL); N, normality of sodium thiosulfate solution (0.01 N); W, weight of sample (g).
Free fatty acid value determination
The free fatty acid (FFA) value of the ready-to-cook soup mix was determined as per the method of Rukunudin et al. [1998] with slight modification. To the ready-to-cook soup mix (5 g), 30 mL of chloroform was added. The suspension was homogenized for 1 min and filtered to remove solid particles. A few drops of the ethanolic phenolphthalein (1%) indicator were added to the filtrate which was then titrated with 0.01 N ethanolic potassium hydroxide (KOH) solution. The free fatty acid percentage was calculated according to formula (5).
Color analysis
The color of the soup was assessed using an NS810 colorimeter (Shenzhen Threenh Technology Co (3nh), Zengcheng, Guangzhou, China) based on the CIELab system (illuminant D65, observer 10°, illumination mode d/8 and caliber 8 mm). The colorimeter was calibrated against black (L*=0, a*=0, b*=0) and white (L*=100, a*=0, b*=0) standard provided with the instrument. The colorimeter was equipped with a light source (combined light-emitting diode sources) and a sensor (silicon photodiode array). The homogeneous RTC soup mix was taken into Petri dish and the parameters of color (L*, a*, b*) were measured on the surface of the soup at three different locations. L* denoted lightness, a* – redness (positive values) or greenness (negative value), and b* – yellowness (positive values) or blueness (negative value).
Microbiological analysis
The microbiological analysis of the ready-to-cook soup mix was performed using a spread plate technique as per the method of Tolasa et al. [2012]. The ready-to-cook soup mix (1 g) was suspended in 10 mL of peptone water (0.1%, w/v) and serially diluted from 10−1 to 10−4 dilution. An aliquot of 100 μL of the sample from different dilutions was plated on different agar plates by the spread plate technique. Nutrient agar, Czapek dox agar, XLD agar and EMB agar media were used for total plate count, yeast and mold count, Salmonella count and Escherichia coli count, respectively. The plates were incubated for 24 h at 37°C for total plate count, Salmonella and E. coli counts and at 28°C for 3 days for yeast and mold counts. Total plate count was calculated using the formula (6).
Results were expressed as log of colony-forming unit (cfu) count per g.
RESULTS AND DISCUSSION
In our previous study, we optimized the microencapsulation of pink perch head and viscera protein hydrolysate using maltodextrin, sodium alginates, gum Arabic and carboxyl methyl cellulose as wall materials [Kumari et al., 2023]. The best microencapsulates (maltodextrin, sodium alginate and gum Arabic in a 1:1:1 ratio, w/w/w) had 76% encapsulation efficiency with improved physiochemical properties compared to the pink perch head and viscera protein hydrolysate. The obtained microencapsulated protein hydrolysate had smooth surface and amorphous structure with no fissures, crack and disruption which retained the antioxidant activity of protein hydrolysate [Kumari et al., 2023]. The microencapsulation of protein hydrolysate reduces its bitter taste, hygroscopicity, and fishy odor, and aids in the controlled release of hydrolysates with time under gastrointestinal conditions. In the present study, we utilized microencapsulated protein hydrolysate for the preparation of ready-to-cook proteinenriched soup mix.
Chemical composition
The nutritional composition of RTC soup mixes depends on the type of ingredients used. The proximate analysis of the soup mix powder gives an idea about nutritional composition and nature of the products. In this study, the proximate composition of RTC soup mixes was given in Table 2. The moisture content of RTC soup mixes was in the range of 4.2 g/100 g to 4.6 g/100 g. However, there was no significant difference (p≥0.05) between the moisture content of different RTC soup mixes. Statistically, the RTC MPHS, PHS, and FPS mixes contained more proteins (p<0.05) than commercial chicken and vegetable soups, i.e., approximately ≥15%. The ash content of RTC MPHS mix was the lowest among all soup mixes, which indicated the presence of low amounts of minerals. Öztürk et al. [2019] reported comparable proximate composition of powdered soups prepared from different species of fish, which contained 28–30% protein, 5–7% fat, 8–10% moisture, 6–7% ash, and 40–50% carbohydrate. Moisture plays an important role in food quality because moist foods deteriorate due to growth of mold and clustering. The chemical composition of all the soups showed that their moisture content was under the limits of Food Safety and Standard Authority of India (FSSAI) standard for soup powder, which is set to be 5.0% [FSSAI, 2022].
Table 2
Chemical composition of ready-to-cook (RTC) soup mixes (g/100 g).
[i] Results are shown as mean ± standard deviation (n=2). Different lowercase letters in column indicate significant differences between RTC soup mixes (p<0.05). BS, blank soup; MPHS, microencapsulated protein hydrolysate soup; PHS, protein hydrolysate soup; FPS, sun dried whole fish powder soup; CCS, commercial chicken soup; CVS, commercial vegetable soup.
Physical properties of RTC soup mixes
Solubility
Solubility affects sensory attributes, such as the taste, in the final products and consumption characteristics of powder products [Azizpour et al., 2016]. The solubility of the RTC soup mixes was between 41.0 to 43.0% (Table 3) and there were no statistically significant differences (p≥0.05) between the RTC soup mixes. In the RTC soup mixes, soluble ingredients were: microencapsulated protein hydrolysate, protein hydrolysate, milk solid, salt and sugar; whereas insoluble ingredients were: vegetables and oil; and partially soluble ingredients were: sun-dried whole fish powder, potato starch, refined flour and spice mix, which affected the solubility of products. Taşkin & Savlak [2022] reported significantly lower solubility of instant soup powders reaching 18–23%. Ready-to-cook soup mixes are supposed to have high water solubility as they must dissolve quickly and be ready to drink in a short time.
Table 3
Physical parameters of ready-to-cook (RTC) soup mixes.
[i] Results are shown as mean ± standard deviation (n=2). Different lowercase letters in column indicate significant differences between RTC soup mixes (p<0.05). BS, blank soup; MPHS, microencapsulated protein hydrolysate soup; PHS, protein hydrolysate soup; FPS, sun dried whole fish powder soup; CCS, commercial chicken soup; CVS, commercial vegetable soup.
pH and total soluble solid content
The pH values of the RTC soup mixes varied between 6.0 and 6.8 (Table 3). Statistically, there was no significant difference (p≥0.05) between pH values of different RTC soup mixes. In the RTC soup mixes, sunflower oil and protein hydrolysates might have got hydrolyzed into free fatty acids and free amino acids, respectively, which may affect the pH of soups. The total soluble solid is defined as the amount of sugar and soluble minerals in the sample [Beckles, 2012]. The TSS of the RTC soup mixes was in the range of 5.5 to 6.8°Brix (Table 3), which was in line with the FSSAI limit for soup powder (more than 5°Brix) [FSSAI, 2022]. The total soluble solid is one of the parameters which affect the sensory properties (taste) of the food. TSS also has a significant effect on the storage stability of products because it depends on the sugar content.
Bulk and tapped density
The flow characteristics of the soup are important to determine the transportation condition, processing condition, quality, and structural behavior of the products [Kumari et al., 2023]. The flowability of powder was determined as the Hausner ratio (HR) and the compressibility index (CI). Flowability of RTC MPHS mix and RTC PHS mix was poor with HR of 1.44 and 1.43, respectively, which was similar to that of the CVS mix (1.35) (Table 3). The poor flowability was observed in all tested soup mixes, which indicated high difference between bulk density and tapped density values. The bulk density of CCS mix was the highest (p<0.05) among all the soup mixes, probably due to the presence of meat shreds in the soup mix. Fasogbon & Taiwo [2019] reported that dika kernel powder soups had poor flowability with HR and CI values in ranges of 1.1 to 1.4 and 10.6 to 30.0, respectively. The HR and CI depend on moisture content of products.
Viscosity
Viscosity of the RTC soup mixes decreased with increase in shear rates (Figure 1). The curves of shear stress vs. shear rate were also prepared (Figure 1B). Results shown that all the RTC soup mixes exhibited non-Newtonian pseudoplastic behavior. Previous studies also reported that different soups (such as tomato, vegetable, gbegiri, ewedu, karkashi and ogbano) showed non-Newtonian pseudoplastic behaviors [Fasogbon & Taiwo, 2019]. The mouthfeel (thickness) is mostly linked to the viscosity of the food products [De Wijk et al., 2003]. The thickness of food products is directly proportional to the shear stress at the surface of the tongue [Thomazo et al., 2019]. At a given stress, deformation rate is higher in low viscous products as compared to the high viscous ones. Therefore, shear stress was used to predict the thickness of products [Abson et al., 2014]. In this study, the RTC MPHS mix was a low viscous product, which can be easily detectible with better mouth feel among other RTC soup mixes with the fish-derived additive.
Pasting properties
The pasting characteristics of different RTC soup mixes were presented in Table 4 and Figure S2. The RTC soup mixes attained their peak viscosity within 8.0–8.5 min. There was a significant (p<0.05) difference between peak viscosity of different RTC soup mixes. The peak viscosity of CVS (1,928.0 mPa×s) and CCS (1,577.0 mPa×s) was higher compared to RTC MPHS mix (293.8 mPa×s) and RTC FPS mix (291.3 mPa×s). This is due to the presence of a thickening agent in CVS and CCS mixes. The pasting temperature of the RTC MPHS mix was the lowest (64.7°C) among all the RTC soup mixes, although the value for FPS was not significantly (p≥0.05) different. A lower peak viscosity as well as final viscosity of the RTC MPHS mix compared to other soup mixes could be due to the presence of maltodextrin, gum Arabic and sodium alginates which enable the powder to rehydrate and form crosslinks. The RTC MPHS mix showed low breaking down viscosity (24.9 mPa×s) followed by RTC PHS mix (131.3 mPa×s), RTC FPS mix (137.2 mPa×s), RTC BS mix (284.9 mPa×s), CVS (767.3 mPa×s), and CCS (914.5 mPa×s). Generally, the presence of protein and starch increases the viscosity [Wang et al., 2020]. However, in this case, the RTC soup mix contains microencapsulated protein hydrolysates which do not release peptides even after heating after 100°C. The protein hydrolysates are released from microcapsules under gastric conditions as per our previous study [Kumari et al., 2023]. Therefore, the RTC MPHS mix showed lower peak viscosity. Hanan et al. [2020] also reported the low pasting properties, including peak viscosity, final viscosity and break down viscosity, of a soup upon the incorporation of pea pod powder, gaur gum and locust bean gum.
Table 4
Pasting characteristics of ready-to-cook (RTC) soup mixes.
[i] Results are shown as mean ± standard deviation (n=2). Different lowercase letters in column indicate significant differences between RTC soup mixes (p<0.05). BS, blank soup; MPHS, microencapsulated protein hydrolysate soup; PHS, protein hydrolysate soup; FPS, sun dried whole fish powder soup; CCS, commercial chicken soup; CVS, commercial vegetable soup.
Consumer assessment
The results of consumer assessment of appearance, taste, odor, flavor and overall acceptability of the RTC soup mixes are shown in Table 5. The RTC soup mixes exhibited overall acceptability scores ranging between 4.5 to 7.6 over the 9-point hedonic scale. The RTC soup mix formulated with microencapsulated protein hydrolysate was more preferred by the assessors among all soup mixes with overall acceptability of 7.6 and with more acceptable appearance, odor, taste and flavour among other soup mixes with the fish-derived additive. There was no significant (p≥0.05) difference between the overall acceptability of the RTC MPHS mix and instant commercial chicken soup. Among all RTC soup mixes, PHS and FPS mixes had significantly (p<0.05) lower overall acceptability scores due to fishy odors and bitter tastes. These results prove the theory that negative attributes like fishy odor and bitterness originated from fish ingredients can be masked by the technology of microencapsulation.
Table 5
Consumer assessment of ready-to-cook (RTC) soup mixes.
[i] Results are shown as mean ± standard deviation (n=30). Different lowercase letters in column indicate significant differences between RTC soup mixes (p<0.05). BS, blank soup; MPHS, microencapsulated protein hydrolysate soup; PHS, protein hydrolysate soup; FPS, sun dried whole fish powder soup; CCS, commercial chicken soup; CVS, commercial vegetable soup.
Storage stability of ready-to-cook soup mixes at accelerated shelf-life testing
The chemical composition, microbial contamination, storage conditions and type of packaging play an important role in the shelf-life of food products [Zarehgashti et al., 2019]. In our study, based on consumer assessment results, it was decided to evaluate the shelf-life of only RTC MPHS mix as the most promising formulation with the fish-derive additive. In addition, the shelf-life of RTC BS and CCS mixes (as controls) was estimated in ASLT (40°C, 75% RH). One day of ASLT condition is equivalent to 4 days of storage at room temperature [Subramaniam, 2009]. The aim of ASLT is to increase the rate of deterioration of the products without altering the mechanism of changes seen in the products under normal storage conditions (25–30°C). Estimation of shelf-life was an important part of the study for any food product formulation to keep the product safe for long period of time and to its further commercialization [Hemanth et al., 2020]. The spoilage factors chosen for the study were oxidation of lipids (evaluated by TBARS level, and peroxide value, and free fatty acid content), changes in pH and color, and microbiological quality.
pH
The pH values of soup mixes are shown in Table 6. There was statistically significant (p<0.05) relationship between the storage time and the pH value. The pH value of the RTC BS mix decreases from 6.5 to 5.3. Whereas there was a slow decrease in pH in the case of RTC MPHS and CCS mixes during storage. The pH value of RTC soup mixes decreases due to chemical and microbial degradation. The pH of soup alone cannot be used as an indicator of soup quality. Therefore, other parameters should be determined along with the pH during storage. Mol [2005] also reported that the pH value of fish soup decreased during storage.
Table 6
Lipid quality parameters of ready-to-cook soup in accelerated shelf-life testing.
[i] Results are shown as mean ± standard deviation (n=2). Different lowercase letters in column indicate significant differences between storage days (p<0.05). BS, blank soup; MPHS, microencapsulated protein hydrolysate soup; CCS, commercial chicken soup; TBARS, 2-thiobarbituric acid reactive substances.
Free fatty acid content
Amount of free fatty acids is a quality indicator in the foods. In this study, day 6 of ASLT (equivalent to 24 days of storage at room temperature) was the beginning of most changes in the FFA content of the RTC soup mixes (Table 6). The FFA of RTC soup mixes significantly increased (p<0.05) during storage. Zarehgashti et al. [2019] studied the shelf-life of ready-to-eat shrimp soup powder and showed that its FFA content increased slowly during 6-month storage at room temperature).
Peroxide value
The peroxide value is used to determine the primary products (hydroperoxides) of lipid oxidation and mostly to detect oxidative rancidity in the products. In this study, the PV of all RTC soup mixes significantly (p<0.05) increased with days of ASLT, as shown in Table 6. The PV of the RTC BS mix increased from 2.07 to 6.67 meq/kg, that of RTC MPHS mix increased from 1.57 to 5.27 meq/kg, and that of CCS changed from 1.37 to 8.63 meq/kg. Zarehgashti et al. [2019] reported that the peroxide value of ready-to-eat shrimp soup powder increased from 0.09 meq/kg to 3.14 meq/kg after storage at room temperature within 6 months.
2-Thiobarbituric acid reactive substance content
During storage, meat-related products are prone to oxidative change. The TBARS estimation is the most widely used method for determination of lipid oxidation. The TBARS value of the RTC soup mixes significantly (p<0.05) increased during storage period, as given Table 6. The highest TBARS level after 15 days of ASLT has been found in RTC BS mix (4.4 mg malondialdehyde/kg) followed by commercial soup mix (4.3 mg malondialdehyde/kg) and RTC MPHS mix (3.9 mg malondialdehyde/kg). The lipid oxidation in RTC MPHS mix was low among all RTC soup mixes probably due to antioxidant activity of protein hydrolysates, which commercial chicken soup mix and RTC BS mix did not contain. The antioxidant properties of fish protein hydrolysates are documented in literature [Noman et al., 2022]. Sarkar et al. [2019] reported that the TBARS value of an instant soup mix blended with spent hen meat shred increased during storage from 0.37 to 0.42 mg malondialdehyde/kg. Öztürk et al. [2019] determined TBARS levels of fish powdered soups during storage at 65°C and 75% RH and found that they increased on average from 2.4 to 7.2 mg malondialdehyde/kg after 6-month storage.
Color
Color is a very important quality parameter because market acceptability, desirability and final price of food products mainly depend on their appearance and color [Azizpour et al., 2016]. The color parameters of stored RTC soup mixes are presented in Table 7. The color of RTC BS mix and RTC MPHS mix at initial day of storage was greenish yellow with L* of 83.5 and 74.2, respectively, a* of 0.04 and 0.9, respectively, and b* of 12.5 and 15.3, respectively. The negative value of a* indicates that the soup was more green in color. Statistically, there were significant (p<0.05) changes in color parameters of RTC soup mixes during storage (Figure S3). With increase in the number of days of ASLT, the color of RTC soups mixes became darker (moves towards reddish zone). It could be caused by chemical changes during ASLT storage. The results of this study indicated that the RTC MPHS mix was assumed to be more attractive to consumers as compared to CCS.
Table 7
Color parameters of ready-to-cook soup mixes in accelerated shelf-life testing.
[i] Results are shown as mean ± standard deviation (n=3). Different lowercase letters in column indicate significant differences between storage days (p<0.05). BS, blank soup; MPHS, microencapsulated protein hydrolysate soup; CCS, commercial chicken soup; L*, lightness; a*, redness (positive values) or greenness (negative value); b*, yellowness.
Microbiological quality
Microbiological quality of RTC soup mixes was studied in respect to total plate count, yeast and mold count, E. coli count, and Salmonella count during storage at accelerated conditions. The results are given in Table 8. There was significant (p<0.05) increase in microbial growth during storage for all types of mixes. This could be due to the gradual increase in the moisture content of samples. Total plate count and yeast and mold count were under the consumption limit of FSSAI standard for fish products (which is 1.0× 106 cfu/g and 1.0×104 cfu/g, respectively [FSSAI, 2022]) till 6 days of storage under accelerated conditions which was equivalent to 24 days of storage at room temperature. Total plate count and yeast and mold count become uncountable at 9 days of ASLT, which indicated that the quality of RTC soup mixes started decreasing due to microbial spoilage. E. coli and Salmonella were not detected in all RTC soup mixes, which indicated that pathogenic bacteria were absent in all RTC soup mixes. The results of this study showed a positive correlation between changes in chemical parameters and microbial spoilage. This finding suggests that the RTC MPHS soup mix was stable and safe for consumption till 24 days at room temperature. The shelf-life of the product could be enhanced with proper sterilization techniques and packaging. Jayasinghe et al. [2016] reported that seaweed-based soup mix powder had shelf-life up to 3 months at ambient temperature when packed in airtight polystyrene packets.
Table 8
Microbiological quality of ready-to-cook soup mixes in accelerated shelf-life testing.
CONCLUSIONS
The study proves that microencapsulated protein hydrolysate, originated from fish processing by-products, can be used as a value-added ingredient for the formulation of RTC soup mixes that have partial replacement potential for products commonly used by the food industry. Soup fortification with peptides and amino acids (15% protein equivalent) by incorporating microencapsulated protein hydrolysate obtained from pink perch by-products, represents a promising strategy to increase the nutritional value of ready-to-cook soup mixes. The developed RTC soup mixes will be an option especially for individuals suffering from protein deficiency. It can be concluded that microencapsulated protein hydrolysate can be effectively used to produce ready-to-cook soup mixes with improved nutritional and overall acceptability scores. The microencapsulated protein hydrolysate increased the overall acceptability of RTC soup mixes by masking bitter taste and fishy odor of protein hydrolysate. The ready-to-cook soup was suitable to be aerobically stored in metallized polyethylene zip lock bag (32-micron thickness) at 60°C and 75% RH for the period of 6 days in ASLT which is equivalent to 24 days of storage at room temperature without any deterioration in its quality and acceptability. The chemical analysis of RTC soup mixes showed that the RTC MPHS mix was at the same level or even better than commercial soups.

