INTRODUCTION
Avocado (Persea Americana Mill.), a subtropical fruit native to Mexico, has recently gained popularity as a cultivated crop in northern Thailand. Per 100 g of fresh pulp, avocado provides approximately 15.40 g of lipids, 8.64 g of carbohydrates, 6.80 g of dietary fiber (about 70% insoluble and 30% soluble), and 1.96 g of proteins, making it a valuable energy source [Ford et al., 2023; Krumreich et al., 2018]. Avocado is particularly rich in monounsaturated fatty acids (approximately 9.80–12.37 g/100 g fresh pulp), along with polyunsaturated (about 1.82–4.46 g/100 g fresh pulp), and saturated fatty acids (around 2.13–3.18 g/100 g fresh pulp) [Ford et al., 2023; USDA, 2011]. Besides being high in lipids with monounsaturated fatty acids, avocado pulp contains a variety of essential nutrients and bioactive compounds, including minerals (potassium, sodium, magnesium), vitamins (A, C, B6 and K, folate, niacin, pantothenic acid, riboflavin, choline and tocopherols), carotenoids (mainly lutein and/or zeaxanthin), phytosterols, phenolic compounds, organic acids and acetogenins as well as exhibits antioxidant, anti-inflammatory, anticancer, and antimicrobial properties, all of which contribute to its broad potential health benefits [Dreher & Davenport, 2013; Ford et al., 2023; Krumreich et al., 2018]. Due to its beneficial lipid composition, avocado consumption can help lower total cholesterol, low-density lipoprotein (LDL) cholesterol, and triglyceride levels, while increasing high-density lipoprotein (HDL) cholesterol, thereby supporting a healthier blood lipid profile [Mahmassani et al., 2018]. Thus, freeze-dried avocado shows promise as an ingredient for replacing fat or flour, while also enhancing the fiber content and nutritional value of food products [Cortés Rodríguez et al., 2019; Moolwong et al., 2023].
Milk tablets are compact, chewable forms of milk powder offering a convenient, portable, and shelf-stable source of essential nutrients such as calcium, protein, and vitamins. However, despite their convenience, traditional milk tables are high in sugar posing health risks such as diabetes, obesity, and high blood pressure when consumed excessively [Endy et al., 2024]. Consuming milk tablets enriched with high levels of dietary fiber and phytochemical from fruits and vegetables significantly contributes to a healthy diet. Therefore, developing healthier milk tablets by partially replacing milk powder with avocado pulp powder could help reduce the risk of chronic diseases and enhance nutritional values in small doses, working best when used as a supplement or snack especially for children, or individuals with limited access to fresh milk. From a production perspective, the use of avocado pulp powder in milk tablets can improve texture, mouthfeel, and sensory attributes, potentially opening new avenues for value-added dairy and plant-based products. This innovation aligns with industry trends toward clean-label, nutrient-dense, and minimally processed foods, providing manufacturers with opportunities to differentiate their products in a competitive market. Thus, this study aimed to optimize the substitution ratio of milk powder with avocado pulp powder in fortified milk tablets and evaluate its impact on physical properties and sensory acceptability, with the goal of developing a functional, health-promoting milk tablet enriched with dietary fiber and antioxidant phytochemicals.
MATERIALS AND METHODS
Materials and reagents
Avocados (Persea Americana Mill.), milk powder, and icing sugar were sourced from a local market in Bangkok, Thailand. Foodgrade CAB-O-SIL and magnesium stearate were obtained from Krungthepchemi Co., Ltd., Bangkok, Thailand. The chemicals: 2,2’-azino-bis-3-ethylbenzthiazoline-6-sulphonic acid (ABTS), 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical, iron(III) chloride hexahydrate, 2,4,6-tripyridyl-s-triazine (TPTZ), 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), and gallic acid were procured from Sigma Aldrich (St. Louis, MO, USA). Folin-Ciocalteu reagent was acquired from Fisher Scientific (Pittsburgh, PA, USA). All culture media for total plate count, mold and yeast count and Escherichia coli count were purchased from HiMedia Laboratories (Mumbai, India). All other materials utilized were of analytical quality.
Preparation of avocado pulp powder
Freeze-dried avocado pulp powder was prepared following the method outlined by Chuacharoen et al. [2021]. Briefly, ripe avocado pulp was blended with added maltodextrin in amounts of 0 g, 10 g, 20 g, and 30 g per 100 g of dry matter (d.m.) of pulp and homogenized using a homogenizer (T10 Ultra-turrax, IKA-Labortechnik, Staufen, Germany) at 500 rpm for 5 min. The resulting avocado paste was then frozen in static air at −80°C and freeze-dried in a laboratory-scale freeze dryer (Christ Alpha 1-2LD Plus, GmbH, Osterode am Harz, Germany) at −50°C for 72 h under a pressure of less than 0.11 mbar. The freeze-dried avocado pulp powder was ground using a rotor mill (FRITSCH P-14, Franconia, Germany) and stored in a desiccator until used.
Preparation of avocado-fortified milk tablets
In the fortified milk tablet formulations, 15% (A15), 25% (A25), and 35% (A35) of the milk powder was replaced by weight with avocado pulp powder incorporated with 30% maltodextrin (Table 1). A control sample without avocado (A0) was also prepared. Other tablet ingredients included CAB-O-SIL added to improve powder flow, content uniformity, and overall tablet quality, and magnesium stearate, acting primarily as a lubricant and secondarily as a mineral supplement [Binte Abu Baker et al., 2019]. All dried ingredients were sifted using a 0.5 mm sieve and mixed in a dry blender until completely mixed. The obtained dried powder was sampled to test flow rheology. Subsequently, the tableting of mixture powders was performed using a laboratory-scale single-punch tablet machine (Yiao-heng Co., Ltd., Bangkok, Thailand), and the resulting tablets were kept in an aluminum foil bag and stored at −18°C for further analyses.
Table 1
Formulations of control milk tablet (A0) and milk tablets containing different levels of avocado pulp powder (A15–A35).
| Ingredient | A0 (control) | A15 | A25 | A35 |
|---|---|---|---|---|
| Milk powder | 70.00 | 59.50 | 52.50 | 45.50 |
| Avocado pulp powder | 0.00 | 10.50 | 17.50 | 24.50 |
| Icing sugar | 28.00 | 28.00 | 28.00 | 28.00 |
| CAB-O-SIL | 1.50 | 1.50 | 1.50 | 1.50 |
| Magnesium stearate | 0.50 | 0.50 | 0.50 | 0.50 |
Analysis of physical parameters
Before tableting, all powder formulations were assessed for bulk density and tapped density. After tableting, the physical properties of the avocado-fortified milk tablets were examined for the uniformity of weight, thickness, hardness, density, and friability. Bulk and tapped densities were determined using a tap density tester (Electrolab India Pvt. Ltd., Mumbai, India). In summary, 100 g of the powder mixture were weighed and poured into a 250 mL measuring cylinder. Bulk density was calculated by dividing the sample weight by its initial volume. The sample was then tapped 200 times to obtain its final volume, which was used to calculate the tapped density by dividing the sample weight by the final volume [Salum et al., 2022]. The calculated bulk density and tapped density values were used to categorize flowability using the Carr’s index (Equation 1) and Hausner ratio (Equation 2), respectively.
Twenty tablets from each formulation were individually measured for weight, thickness, and diameter. The tablet weight was measured using a high-precision laboratory balance (Sartorius CP32025, Sartorius AG, Göttingen, Germany). Their thickness and diameter were measured using a vernier caliper. The tablet hardness was measured using a texture analyzer (TA.XT.Plus, Stable Micro System, Godalming, Surrey, UK) in a compression mode (heavy duty platform/confectionery holder; HDP/CH) with a P/6 probe (pre-test speed: 1 mm/sec, test speed: 2 mm/sec, post-test speed: 10 mm/sec). The measurement was repeated 10 times, and the results were recorded as the hardness at the highest force, expressed in N.
For tablet density, six tablets were randomly selected, and their individual weights were recorded using a digital scale. Chloroform was used, and the solution was poured into a graduated cylinder. The volume change of the liquid inside the cylinder was recorded. The density was then calculated as the ratio of its mass to the displaced volume (g/mL), and results were expressed as mean and standard deviation (n=6).
The friability is a key parameter for quality assessment. Twenty tablets were initially dusted using a dusting brush and weighed to record their initial weight. They were then placed in a friabilator (Roche Model PTFR-A, Pharmatest, Germany) and rotated at a speed of 25 rpm for 4 min. Subsequently, the tablets were removed, dedusted, and reweighed to determine their final weights. The friability (%) was calculated based on the weight difference before and after rotation (Equation 3).
Color measurements were performed using a MiniScan XE Plus colorimeter (Hunterlab, Reston, VA, USA). The instrument was calibrated prior to measurement using a standard white calibration tile and a black calibration plate according to the device manufacturer’s instructions. The L*, a*, and b* values were recorded, where L* denotes lightness, ranging from 0 (black) to 100 (white). The a* coordinate represents the green-red axis, with negative values corresponding to green hues and positive values corresponding to red hues. The b* coordinate corresponds to the blue-yellow axis, where negative values indicate blue hues and positive values indicate yellow hues.
Proximate analysis and water activity measurement
Proximate composition was analyzed according to the standard methods of the Association of Official Analytical Chemists [AOAC, 2019]. Moisture and total solid contents of avocado pulp and milk tablets fortified with avocado pulp powder were determined gravimetrically by drying the sample in a convection oven at 110°C for 2 h (method 968.11). Ash content of avocado-fortified milk tablets was quantified by incinerating the samples at 550°C in a muffle furnace (method 945.38). Crude protein was determined using the Kjeldahl digestion method (method 971.09), while crude fiber was analyzed through sequential acid and alkaline digestion (method 962.09). To determine total lipid content, lipids were extracted using the Soxhlet extraction method with n-hexane as the solvent in a Soxtec Foss apparatus (ST 255 SoxtecTM, FOSS Analytical Solutions Pty. Ltd., Victoria, Australia) at 120°C for 80 min (method 920.39). Water activity (aw) was measured using an AquaLab 4TE meter (Meter Group Inc., WA, USA).
Determination of total phenolic content and antioxidant capacity
To determine total phenolic content (TPC) and antioxidant capacity of avocado-fortified milk tablets, the samples were extracted following the method of Pongtongkam et al. [2024] with slight modifications. In brief, approximately 0.5 g of avocado milk tablet was extracted using 50 mL of 80% (v/v) methanol, followed by mechanical shaking using an incubator (C25KC incubator shaker, New Brunswick Scientific, NJ, USA) at 40 rpm and room temperature for 4 h. The mixture was then centrifuged using an MPW-352R centrifuge (MPW Med. Instruments, Warsaw, Poland) at 1,277×g for 30 min. The resulting supernatant was collected and stored at −20°C for further analyses.
Total phenolic content
The total phenolic content (TPC) was measured using the Folin-Ciocalteu reagent by Singleton et al. [1999] method with minor modifications. In brief, 20 μL of the extract, gallic acid standard, or blank sample were transferred into separate test tubes and mixed with 1.58 mL of distilled water. Subsequently, 100 μL of Folin–Ciocalteau reagent were added, and the mixture was thoroughly vortexed. Within 8 min, 300 μL of a 7.5% sodium carbonate solution were introduced, and immediately mixed. The samples were then incubated in the dark at room temperature for 30 min. Absorbance was recorded at 765 nm using a UV-Vis spectrophotometer (Spectronic GENESYS2, Thermo Fisher Scientific, Waltham, MA, USA). The TPC was calculated from a gallic acid calibration curve, with results expressed as μg of gallic acid equivalent per g of milk tablet (μg GAE/g).
Antioxidant capacity
The antioxidant capacity of the avocado milk tablets was evaluated using three in vitro antioxidant assays – 1,1-diphenyl-2-picrylhydrazyl (DPPH), 2,2’-azino-bis-3-ethylbenzthiazoline-6-sulphonic acid (ABTS), and ferric reducing antioxidant power (FRAP).
The DPPH assay was performed according to the method of Brand-Williams et al. [1995] with slight modifications. Briefly, 200 μL of the extract was mixed with 2.8 mL of a 100 μM DPPH radical solution prepared in methanol and incubated in the dark for 30 min. A control containing the DPPH radical solution and 80% (v/v) methanol was also prepared. The absorbance was measured at 515 nm using a UV-Vis spectrophotometer (Spectronic GENESYS2, Thermo Fisher Scientific, Waltham, MA, USA). A standard curve was plotted using Trolox standard solutions at various concentrations in 80% (v/v) methanol. Results were expressed as μmol of Trolox equivalent per g of milk tablet (μmol TE/g).
The ABTS assay was carried out following the method of Re et al. [1999] with slight modifications. Briefly, the ABTS•+ was generated by dissolving 7 mM ABTS in 2.45 mM potassium persulfate, and the mixture was left in the dark at room temperature for 15 h before use. The resulting ABTS•+ solution was then diluted with 5 mM sodium phosphate buffer (pH 7.4) to achieve an absorbance of 0.70±0.02 at 734 nm, measured using a UV-Vis spectrophotometer (Spectronic GENESYS2, Thermo Fisher Scientific). Subsequently, 50 μL of each sample were combined with 150 μL of the ABTS•+ solution and incubated for 10 min at 25°C. The ABTS•+ scavenging activity was quantified using a Trolox calibration curve, with results expressed as μmol of Trolox equivalent per g of milk tablet (μmol TE/g).
The FRAP assay was conducted according to the method described by Benzie & Strain [1996] with minor modifications. In brief, 100 μL of the extract were mixed with 300 μL of distilled water and 3 mL of FRAP reagent containing a 2,4,6-tri(2-pyridyl)-1,3,5-triazine (TPTZ) solution in 40 mM HCl, acetate buffer (pH 3.6), and ferric chloride solution in a test tube. The mixture was incubated in the dark for 30 min, after which the absorbance of the resulting ferrous tripyridyltriazine colored complex was measured at 593 nm using a UV-Vis spectrophotometer (Spectronic GENESYS2, Thermo Fisher Scientific). The FRAP was determined from a Trolox standard curve, with results expressed as μmol of Trolox equivalent per g of milk tablet (μmol TE/g).
Microbiological evaluation
The microbiological quality was evaluated following the method described by Pongtongkam et al. [2024] with slight modifications. Twenty-five g of milk tablets were weighed and mixed with 225 mL of a 0.1% peptone solution, followed by blending for 2 min. The mixture was then serially diluted to obtain decimal dilutions ranging from 10−1 to 10−4. For total plate count, 1 mL of each dilution was transferred to a sterile Petri dish. Then, molten plate count agar (PCA) was poured over the sample. The plate was gently swirled to mix the sample and agar. All PCA plates were incubated at 35°C for 48 h. Total plate count was computed from colony enumeration. For mold and yeast counts, 1 mL of 10−1 dilution was plated by dividing into 3 aliquots (0.3, 0.3, 0.4 mL each) onto 3 separate potato dextrose agar (PDA) plates. The spread plate technique was then performed on each plate. PDA plates were incubated at 25°C for 5 days. Microbial counts were first calculated as colony-forming units (CFU) per g of milk tablet (CFU/g) and then converted to log10 CFU/g (log CFU/g) for reporting in the results table. For E. coli enumeration, 3 dilutions of 10−1, 10−2 and 10−3 were used for a 3-tube most probable number (MPN) analysis. To confirm the presence of E. coli, all presumptive, confirmed, completed, and IMViC tests were performed. The IMViC series included the indole test (I), methyl red test (M), Voges-Proskauer test (V), and citrate utilization test (C), which together provided characteristic biochemical results indicative of E. coli. The number of sample tubes containing E. coli was used to consult the MPN table for E. coli count (MPN/g). All analyses were performed in triplicate.
Sensory evaluation
A sensory evaluation of the avocado-fortified milk tablet was conducted with 60 untrained panelists due to practical constraints related to participant availability and study logistics. The panel included 27 men and 33 women, aged 18–55 years, representing the target consumer group. Each panelist was presented with one piece of randomly coded samples, labeled with three-digit codes, in a randomized order. Water was provided for cleansing the palate between samples. The sensory attributes were assessed using a 9–point hedonic scale, ranging from 1 (dislike extremely) to 9 (like extremely). The panelists evaluated the tablets based on appearance, color, aroma, flavor, texture, hardness, and overall acceptability [Karaman et al., 2014; Tobin et al., 2013]. The hardness in the instruction sheet was defined as the force required to bite or compress the tablet, using a scale from 1 (very soft: breaks immediately with light pressure) to 9 (very hard: difficult to bite and requires strong pressure).
Statistical analyses
All experiments were carried out in triplicate, and the results are expressed as mean ± standard deviation. Statistical analyses were performed using SPSS software version 26 (IBM, Armonk, NY, USA). One-way analysis of variance (ANOVA) followed by Duncan’s multiple range test was applied to determine significant differences between mean values, with a significance level set at p<0.05.
RESULTS AND DISCUSSION
Avocado pulp powder preparation
Avocado pulp was lyophilized with various maltodextrin contents. The powders produced at 0 and 10 g maltodextrin per 100 g d.m. of pulp were not completely dry, exhibiting a sticky and rubbery texture, making it unsuitable for tablet formulation. In contrast, at 20 and 30 g maltodextrin per 100 g d.m. of pulp, the powder was completely dry, puffy, and bright green, making it ideal for tablet production. The moisture content of the powders with maltodextrin at 20 and 30 g/100 g pulp d.m. did not differ significantly (p≥0.05), whereas the water activity was different (p<0.05), with a lower value found in the avocado pulp powder with maltodextrin at 30 g/100 g pulp d.m. (Table 2). This indicates that the powder at this level was the most suitable for use as an ingredient in tablet formulation. The addition of more maltodextrin resulted also in brighter, greener, and less yellowish avocado pulp powder (Table 2). The physical properties of the powder with maltodextrin at 30 g/100 g pulp d.m. were consistent with our previous findings [Chuacharoen et al., 2021]. In general, the standard moisture content required for dried food products is between 4 to 6 g/100 g, and mixtures with high water content affect the physical properties of tablet formation, particularly the texture and hardness.
Characterization of mixture formulations and avocado-fortified milk tablets
The physical properties of the powder mixtures and milk tablets with substitutions of 0%, 15%, 25%, and 35%, w/w, milk powder with avocado pulp powder are summarized in Table 3. Bulk and tapped densities decreased progressively with increasing avocado content, indicating that higher levels of avocado pulp produced lighter, less dense powders with reduced packing efficiency.
Table 3
Physical properties of formulation mixtures and milk tablets without (A0, control) and with 15% (A15), 25% (A25), and 35% (A35) substitution by weight of milk powder with avocado pulp powder.
Flowability was evaluated using Carr’s index and the Hausner ratio, derived from bulk and tapped densities. Lower Carr’s index values (0–15%) and Hausner ratios near 1.0 indicate excellent to good flow, while higher values reflect progressively poorer flow. Values above 38% for Carr’s index or above 1.60 for the Hausner ratio represent very poor flow [Lebrun et al., 2012; Reddy et al., 2014].
The avocado-fortified formulations showed Carr’s index ranging from 18.0 to 30.8 and Hausner ratio from 1.23 to 1.48 (Table 3), indicating generally fair flowability. Only the formulation with the highest avocado pulp powder content (A35) demonstrated poor flowability. Assessing the Hausner ratio is important for understanding how humidity influences powder cohesiveness over shelf-life, as it indicates transitions between free-flowing and cohesive states [Lumay et al., 2012]. Consistent with these indicators, A15 and A25 showed fair flow characteristics, with no significant difference from the control, while A35 exhibited reduced flow.
The obtained tablets had weight, thickness, and diameter ranges of 2.45 to 2.50 g, 3.99 to 4.22 mm, and 5.07 to 5.33 mm, respectively. Weight uniformity is considered a critical physical characteristic, as it is closely related to the content and consistency of the chemical ingredients. The control milk tablets exhibited the highest hardness (47.73 N), which decreased as the proportion of avocado pulp powder in the formulation increased, and hardness of the tablets with 35% (w/w) substitution of milk powder with avocado pulp powder was 25.78 N (Table 3). Density and friability are important indicators of whether a tablet is suitably formed for consumer acceptability, particularly in terms of chewability. The increased avocado pulp powder content led to a decrease in tablet density, from 0.50 g/mL for control tablets to 0.38 g/mL for A35 sample. This reduction in density was correlated with increased friability (from 7.6% to 20.1%), as lower-density tablets are more prone to breaking. Adding avocado pulp powder (high in lipid and moisture) to milk tablet formulations tends to decrease their hardness and increase friability, mainly because the lipids act as a lubricant, weaken protein-protein bonding, and modify moisture distribution within the compressed structure [Liu, 2023; Chauhan et al., 2020].
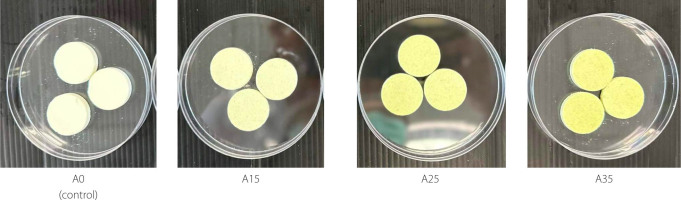
The color of a food product plays an important role in visual appeal, which directly influences consumer acceptance [Moolwong et al., 2023]. The appearance and color parameters of milk tablets containing avocado pulp powder are shown in Figure 1 and Table 3, respectively. Incorporating avocado pulp powder resulted in changes in the color of the tablets. Increasing the avocado powder ratio led to a noticeable decrease in brightness with L* values from 85.59 for A35 sample to 95.48 for control tablets, along with shifts in green hues (decreasing a* values from −1.25 to −3.78) Moreover, the intensity of the yellow hue (b* values) increased significantly (p<0.05). These changes in yellow and green hues were clearly visible to the naked eye (Figure 1).
Figure 1
Images of milk tablets without (A0, control) and with 15% (A15), 25% (A25), and 35% (A35) substitution by weight of milk powder with avocado pulp powder.
Moisture content and water activity may affect the texture, compressibility, and microbial stability of the tablets. As shown in Table 4, an increase in the content of avocado pulp powder in tablets primarily results in their higher moisture content. Water activity (aw) of the milk tablets with 35% (w/w) substitution was 0.46 and this value was significantly (p<0.05) higher than that for control tablets and tablets with other substitution levels (0.43–0.44). The crude protein content of the tablets decreased with an increasing substitution level, because the control’s protein was mainly derived from milk powder, which was partially replaced by avocado pulp powder. This finding is consistent with a study on milk ice cream where milk fat was substituted with fresh avocado pulp [Moolwong et al., 2023]. The total lipid content was significantly increased by partially replacing milk powder with avocado pulp powder (p<0.05) (Table 4). Increasing lipid content with avocado pulp powder can be considered a healthy choice, as avocado is rich in monounsaturated fatty acids and is recognized as a source of healthy fats [Bes-Rastrollo et al., 2008]. Additionally, the lipid fraction in avocado contains lipid-soluble vitamins and tocopherols, making it a valuable ingredient for enhancing the nutritional quality of fat-containing food products [Green & Wang, 2020]. Naturally, avocado pulp powder contains lipids and moisture-retaining compounds, when adding to milk tablets or other milk products, it changes the texture because the lipid droplets from avocado interact with milk proteins, these interactions can make the products either softer or more compact, depending on lipid and moisture contents retained [Pan et al., 2025].
Table 4
Proximate composition and water activity of milk tablets without (A0, control) and with 15% (A15), 25% (A25), and 35% (A35) substitution by weight of milk powder with avocado pulp powder.
Fresh avocado pulp is rich in dietary fiber [Slater et al., 1975; Viera et al., 2023]; thus, its incorporation into the tablets led to a significant increase in crude fiber content, with clear differences observed among the various formulations (p<0.05) (Table 5). The crude fiber content in the tablets containing avocado at 35% (w/w) substitution was three times higher than that of the control. It has been hypothesized that incorporating plant-derived cellulose fibers into food products can enhance their nutritional value. Studies have concluded that enriching foods with dietary fiber is an effective strategy to improve both their nutritional and physiological properties. This observation is consistent with Abraham et al. [2025], who reported that fiber-rich plant powders improve nutritional profiles and reduce carbohydrate density in dairy-based snacks. Ash content of the milk tablets increased with higher avocado pulp powder levels, rising from 1.20 g/100 g in the control to 2.46 g/100 g in the A35 formulation (Table 5), indicating a higher mineral content in the avocado-fortified tablets. Therefore, avocado milk tablets offer a healthier alternative, providing a higher fiber content compared to standard milk tablet formulation. Incorporating dietary fibers from plants not only alters the physical properties of food but also enhances the bioavailability of nutrients and the phytochemical aspects of the food [Unlu et al., 2005].
Table 5
Total phenolic content (TPC), antioxidant capacity, and microbiological properties of milk tablets without (A0, control) and with 15% (A15), 25% (A25), and 35% (A35) substitution by weight of milk powder with avocado pulp powder.
Total phenolic content and antioxidant capacity of avocado milk tablets
Total phenolic content of the avocado milk tablets was determined using the method with Folin-Ciocalteu reagent, which relies on a redox reaction [Berker et al., 2013]. Antioxidant capacity was evaluated using DPPH, ABTS and FRAP assays. The DPPH assay operates through both single electron transfer (SET) and hydrogen atom transfer (HAT) mechanisms, in which antioxidants neutralize the DPPH radical either by donating an electron or a hydrogen atom, respectively. This dual mechanism allows DPPH to detect a broad range of radical-scavenging compounds and is particularly sensitive to lipophilic antioxidants [Yamauchi et al., 2024]. In contrast, ABTS and FRAP assays are more effective for antioxidants with higher polarity, as they primarily measure electron transfer reactions in aqueous systems [Knez et al., 2025; Martysiak-Żurowska & Wenta, 2012]. TPC and antioxidant capacity of the tablets containing avocado pulp powder were significantly higher than those of the control tablets without avocado (p<0.05) (Table 5). This was expected, because, as indicated by literature data, avocado pulp powder has high TPC and antioxidant potential in ABTS, DPPH, and FRAP assays [Chuacharoen et al., 2021; Nguyen et al., 2022, 2023]. The TPC of the avocado-incorporated tablets ranged from 0.73 to 1.91 μg GAE/g, increasing with higher avocado pulp powder ratios. The antioxidant capacity of the fortified tablets also significantly (p<0.05) increased with increasing levels of substitution of milk powder with avocado pulp powder and ranged from 1.78 to 3.84 μmol TE/g in DPPH assay, from 0.92 to 1.38 μmol TE/g in ABTS assay, and from 8.93 to 9.24 μmol TE/g in FRAP assay. The increased total phenolic content and antioxidant capacity observed at higher avocado levels align with the findings of Moolwong et al. [2023], who reported concentration-dependent increases in DPPH scavenging activity when avocado powder was incorporated into milk-based functional ice cream. Similarly, studies by Novelina et al. [2022], Santos et al. [2024], and Siol & Sadowska [2023] reported that avocado pulp and seed flours could significantly enhance the fiber and phenolic content of baked products, thereby improving their antioxidant capacity. These results collectively indicate that the incorporation of fruit-derived bioactive ingredients, such as avocado pulp powder, can effectively enrich the TPC and antioxidant properties of food matrices.
Microbial quality of avocado milk tablets
Microbiological qualities of avocado milk tablets are displayed in Table 5. In terms of the total plate count, a continuous rise in microbial numbers was observed in the samples with an increasing content of avocado pulp powder. The tablets with the avocado ratio of 35% (w/w) had the highest microbial load of 3.00 log CFU/g (p<0.05). However, this level of microbes was within the microbiological specification (6 log CFU/g) set by the Thai Industrial Standards Institute [2007], according to the Thai Community Product Standard 1405/2550. Similarly, the maximum mold and yeast count of 1.89 log CFU/g was detected in the tablets with 35% (w/w) substitution with avocado pulp powder (p<0.05), which was within the acceptable limit according to the specification (2 log CFU/g). Regarding the E. coli count, E. coli was absent in all sample tubes analyzed by the MPN method, with results reported as <3 MPN/g, fully meeting the specification (<3 MPN/g). Nevertheless, the presence of some microorganisms in the avocado milk tablets is most likely attributable to the avocado pulp powder quality, as the freeze-drying process used for the avocado is less lethal to microbial cells compared to traditional thermal processing [Bourdoux et al., 2016]. Contamination by aerobic microorganisms, molds, and yeasts can occur during grinding, ingredient sifting and mixing, and tableting, particularly if the equipment or processing environment is not fully sterile. From a microbiological perspective, the low aw of the avocado milk tablets generally prevents microbial spoilage, provided they are stored in a dry environment.
Sensory scores of avocado milk tablets
Generally, guidelines recommend a minimum of 90 panelists for formal sensory studies; however, smaller sample sizes are commonly used in consumer testing to capture general perceptions of product attributes. This study used 60 untrained panelists because it remained in the product development stage, where a minimum of 60 panelists is considered sufficient for home-use tests employing a 9-point hedonic scale with a 95% confidence interval to assess overall consumer acceptance. Sensory evaluation results for the avocado milk tablets are presented in Table 6. The addition of avocado pulp powder influenced the sensory attributes, with overall acceptability decreasing at higher substitution levels due to the characteristic grassy aroma of avocado. Tablets with substitution of 35% (w/w) of milk powder with avocado pulp powder received the lowest scores for both odor and taste, with the decline in taste scores being statistically significant (p<0.05) compared to A25 formulation. As taste is a key sensory attribute for consumer acceptance, it is notable that no significant difference in taste was observed between the avocado-fortified tablets with 15% and 25% (w/w) substitution, suggesting that up to 25% (w/w) avocado powder can be incorporated without negatively impacting acceptability. Texture and hardness scores also declined as the avocado pulp powder ratio increased, which correlated with physical measurements obtained from texture analysis. This reduction in sensory scores is associated with the higher fiber and lipid content of avocado pulp powder, which affects the structural integrity of the tablets. A 35% inclusion of avocado pulp powder led to a significant decrease in overall acceptability, indicating that this level exceeds the sensory threshold for consumer preference. The control tablets displayed moderate sensory scores across all attributes but were consistently rated lower than the 15% and 25% formulations for color, texture, hardness, and overall acceptability. This indicates that incorporating avocado pulp powder, particularly at 15-25% enhanced consumer perception compared to the control.
Table 6
Sensory scores of milk tablets without (A0, control) and with 15% (A15), 25% (A25), and 35% (A35) substitution by weight of milk powder with avocado pulp powder.
CONCLUSIONS
This study successfully developed a functional food product – milk tablets enriched with freeze-died avocado pulp powder – to address the growing demand for health-oriented foods fortified with bioactive compounds. Incorporation of avocado pulp powder affected both the nutritional composition and the physical characteristics of the tablets. The avocado-enriched formulations showed a higher crude fiber content than conventional milk tablets, attributable to the insoluble fibers and cell-wall components naturally present in avocado pulp powder. A substitution-dependent increase in total phenolic content and antioxidant capacity was also observed. Among the tested formulations, 15% and 25% (w/w) substitution provided the best balance between functional enhancement and sensory acceptability, demonstrating that the bioactive-rich plant ingredient can improve health-promoting properties without compromising consumer acceptance. This suggests that developing avocado milk tablets offers a convenient, shelf-stable way to deliver the combination of nutritional benefits of avocado pulp and milk presenting a promising innovation for the food industry trends towards functional, value-added foods. For consumer perspective, these tablets provide essential nutrients, healthy lipids, and antioxidants in a practical, easy-to-consume form.

