INTRODUCTION
Global aquaculture has increased in recent years and currently provides 50% of the world’s food fish supply [Botta et al., 2020]. In 2020, global mollusc production was over 17.7 million tons of which 6.06 million tons were oysters [FAO, 2022]. In 2018, commercial oyster production in Vietnam was at 15,000 tons from the coastal areas with Crassostrea angulata as the main farmed species [Ugalde et al., 2023]. Oyster production relies on spat collection from the wild but the increasing demand for juveniles has led to the use of hatcheries [Rato et al., 2019]. The contents of lipids, carbohydrates and protein of bivalves are influenced by the dietary nutritional profile [Anjos et al., 2017].
The oyster’s chemical composition varies between species due to factors like season, habitat, sexual maturity, age, and sexual cycle, with season being the most important factor among them [Hurtado et al., 2012]. Lipid reserves in bivalves are an important energy source used in gonad maturation being utilized for energy during gametogenesis and a constituent in larvae and eggs [Rato et al., 2019]. Neutral lipids are the major energy reserve in most marine animals, and their composition depends on the diet source [De La Parra et al., 2005]. Diet (microalgae) has been shown to influence the polyunsaturated fatty acid (PUFA) profile of oysters as most bivalves have limited ability to produce long-chain fatty acids from short-chain precursors [Chu & Greaves, 1991]. Lipids found in the oyster muscle contain high levels of phospholipids with important PUFAs such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). The lipid content and composition of the fish and mollusc muscle vary depending on the fish species, season, sex, sexual maturation, and geographical variations [Kandemi̇r & Polat, 2007]. In oysters, reproductive cycles affect the lipid profile more than the diet. Liu et al. [2020] showed seasonal variations in the lipid profile of the oyster, Crassostrea talienwhanensis, from the Yellow Sea area, with samples harvested in February having higher levels of PUFAs (48.77% of total lipids) and phospholipids (52.12% of total lipids). In turn, the oysters Crassostrea rhizophorae cultivated in the winter had a higher level of PUFAs, particularly the eicosapentaenoic-docosahexaenoic acid combination, and lower levels of saturated fatty acids [Lira et al., 2013].
Pacific oyster Crassostrea gigas is widely cultured in Khanh Hoa province, Vietnam, at three different coastal areas: (1) Thuy Trieu lagoon – Cam Lam district, (2) Nha Phu lagoon – Ninh Hoa district, and (3) Mon lagoon – Van Ninh district. The climatic and environmental characteristics, as well as food system of these coastal areas, are different from each other. This is believed to have an effect on the lipid composition and, consequently, the nutritional value of Pacific oyster muscle. A previous study by Tung & Son [2019] reported that sea surface temperature of central Vietnam exhibited an average value of 23°C from January to February and 32°C in July to August, accompanied by a relatively stable salinity ranging from 31‰ to 34‰. In the mangrove ecosystem of Khanh Hoa province, a study by Lan et al. [2021] revealed a high diversity of phytoplankton, particularly silica algae, with water temperatures ranging from 29.4°C to 30.1°C. Truong et al. [2014] identified 185 zooplankton species in the Nha Phu estuary, with seasonal temperature variations ranging from 25.5°C in the wet season to 32.7°C in the dry season. The objective of this study was to understand the seasonal and locational variations in the lipid profile, including lipid classes, and fatty acid (FA) composition, of C. gigas harvested from Khanh Hoa coast. Results of this study will provide information on when to harvest C. gigas oyster for the highest lipid and the health indices of the lipids, which can be used in dietary recommendations.
MATERIAL AND METHODS
Materials and reagents
Oysters (Crassostrea gigas) were harvested from three Vietnamese farming regions; Thuy Trieu lagoon – Cam Lam, Nha Phu lagoon – Ninh Hoa, and Dam Mon lagoon – Van Ninh at the same location, on the 23rd and 24th of each month for 12 months from January 2021 to December 2021. The samples were commercial quality oysters (after 7–8 months of stocking) with an average size of 65–75 mm in length, and a weight of 70–80 g/oyster. After harvesting, the oysters were washed in seawater, placed in styrofoam boxes with ice, and transported to the laboratories of the Nha Trang University, Vietnam. Oyster muscle was manually separated from the shells, vacuum packed in polyamide bags and frozen at –35±2°C in an air-blast freezer (Seatecco Corporation, Da Nang, Vietnam). Frozen samples were stored at –80°C and analyzed within 2 weeks. Before analysis, the samples were completely thawed in a refrigerator at a temperature of 2°C.
The analytical materials and analytical grade reagents included n-hexane, chloroform, methanol, l-ascorbic acid, thin layer chromatography (TLC) plates (TLC silica gel 60 F254), silica gel 60 (0.040–0.063 mm), diethyl ether, potassium chloride, sodium sulphate, and ninhydrin, and were purchased from Sigma-Aldrich (Burlington, MA, USA). All other reagents were also of analytical grade and were obtained from Merck (Darmstadt, Germany).
Determination of total lipid content
Oysters were extracted to obtain total lipid content according to the method of Bligh & Dyer [1959]. Briefly, 25 g of thawed oyster muscle was mixed with 25 mL of chloroform and 50 mL of methanol and homogenized in an Ultra-Turrax homogenizer (T25 basic, Ika Labortechnik, Staufen, Germany) for 2 min. To this end, 25 mL of chloroform was added, and the mixture was homogenized for 1 min. Then, 25 mL of 0.88% KCl was added to induce phase separation, and the mixture was homogenized for 1 min. After another 1 min of homogenization, the mixture was centrifuged at 1,942×g (Hermle Z326K universal refrigerated centrifuge, Wehingen, Germany) for 20 min at 4°C. The chloroform phase was collected and filtered through Whatman GF/C filters (Cytiva, Buckinghamshire, UK) filled with anhydrous sodium sulphate (Na2SO4) to remove traces of methanol and water. The total lipid content was determined by weighing after evaporation of chloroform to dryness at 40°C in a water bath, using a liquid nitrogen stream. The results were expressed as g per 100 g of the wet weight of oyster muscle.
Phospholipid content determination
Phospholipid (PL) content of the oyster muscle was determined according to the method of Stewart [1980], based on the complex formation of phospholipids with ammonium ferrothiocyanate. Briefly, a 100 μL of an aliquot of the chloroform phase containing lipids, extracted using the Bligh & Dyer method [1959], was added to 2 mL of chloroform. Subsequently, 1 mL of a thiocyanate reagent was added, and the mixture was vortexed for 1 min and centrifuged at 2,000×g (Hermle Z326K centrifuge) for 5 min at 4°C. The absorbance of the lower layer was measured at 488 nm using a Libra S50 UV/VIS spectrophotometer (Biochrom, Cambridge, UK). The results were expressed as g per 100 g of the total lipid and calculated using a standard curve prepared from phosphatidylcholine.
Free fatty acid content determination
Free fatty acid (FFA) content was determined according to the method of Bernárdez et al. [2005], based on complex formation of fatty acids with cupric acetate-pyridine. Briefly, 3 mL of an aliquot of the chloroform phase containing lipids, extracted using the Bligh & Dyer method [1959], was evaporated to dryness at 40°C in a water bath, using a liquid nitrogen stream. Subsequently, 3 mL of cyclohexane and 1 mL of cupric acetate-pyridine reagent were added, and the mixture was vortexed for 1 min and then centrifuged at 2,000×g (Hermle Z326K centrifuge) for 10 min at 4°C. The absorbance of the upper layer was measured at 715 nm using a Libra S50 UV/VIS spectrophotometer (Biochrom). The results were expressed as g FFA per 100 g of the total lipid using a standard curve prepared from oleic acid.
Neutral and polar lipid separation
Total lipids recovered from C. gigas for the months of January, May, September, and November of each location were separated into neutral lipid and polar lipid fractions using column chromatography on silica gel according to the method described by Pernet et al. [2006] with some modifications. A glass column with an inner diameter of 180 mm, a length of 330 mm, and silica gel 60 (0.040–0.060 mm) were used. A lipid to sorbent ratio of 1:50 (w/v) was used, and the column was pre-conditioned with methanol and chloroform. A solution of 0.8 g of the lipid sample in 1 mL of chloroform was loaded onto the column and eluted at a rate of 1 mL/min with chloroform (100%) for 10 min followed by chloroform-methanol (95:5, v/v) for 20 min and then with chloroform-methanol (90:10, v/v) for 30 min to obtain the neutral lipid fraction. The elution was stopped when all the neutral lipids had been collected as confirmed using thin layer chromatography (TLC). Polar lipids were recovered by elution with methanol and complete recovery of the polar lipids was confirmed by performing TLC.
TLC was performed following the method described by Deranieh et al. [2013] with some modifications. TLC silica gel 60 F254 plates were used as the stationary phase. The mobile phase for developing and separating neutral lipids consisted of a mixture of n-hexane/diethyl ether/acetic acid in a ratio of 70:30:2 (v/v/v). Another mobile phase composed of chloroform/methanol/water at a ratio of 65:25:4 (v/v/v) was used for developing and separating polar lipids. After TLC separation, the localization of the neutral lipids was visualized using iodine vapors, while the visualization of polar lipids was achieved using ninhydrin and 10% H2SO4 in MeOH.
The eluents of each fraction were evaporated using a rotary evaporator under vacuum at 40°C (Yamato RE-801-AW2 rotary evaporator, Yamato, Japan) and further under a gentle stream of nitrogen at 35°C. The weight of neutral and polar lipids was recorded, and the fractions were stored at −20°C in the dark until further analysis. The results were expressed as g neutral lipids or g polar lipids per 100 g of the total lipids.
Fatty acid profile analysis
Fatty acids of total lipids extracted were transesterified to methyl esters using base-catalysed esterification according to the American Oil Chemists’ Society (AOCS) official method (Ce 1b-89, 2017) [AOCS, 2017]. The fatty acid methyl esters (FAMEs) were dissolved in iso-octane and injected into a model GC 17A gas chromatograph (Shimadzu Corp., Kyoto, Japan) equipped with a Zebron ZB-wax fused silica wall-coated open tubular column (0.25 mm i.d. × 30 m, 0.25 μm in film thickness; Torrance, CA, USA) and a flame-ionization detector. The column oven and injection port temperature were held initially at 170°C for 2 min, then programmed to 240°C at a rate of 5°C/min, from 240°C to 250°C at a rate of 1.6°C/min, and finally held at 250°C for 10 min. Nitrogen was used as a carrier gas with an inlet pressure of 2.0 kg/cm2. Results were expressed as g per 100 g of the total lipids, and C23:0 (tricosanoic acid) was used as an internal standard.
Thrombogenicity index calculation
Thrombogenicity index (TI) was calculated according to Equation (1) by Ulbricht & Southgate [1991]. TI is the relationship between the pro-thrombogenic saturated fatty acids and the anti-thrombogenic monounsaturated fatty acids (MUFAs) and polyunsaturated fatty acids (PUFAs) that indicates the tendency to form clots in the blood vessels.
Statistical analysis
All experiments were conducted in triplicate. The results were presented as means and standard deviations (SD). All the statistical analyses were carried out using the SPSS (version 26) software (SPSS Inc., Chicago, Illinois). The results were analyzed using one-way analysis of variance (ANOVA). Differences between samples were tested for significance by Student-Newman-Keuls post-hoc test at the 5% level of significance.
RESULTS AND DISCUSSION
Total lipid content
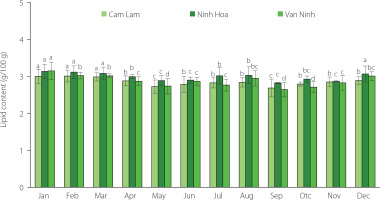
The total lipid content of the muscle of C. gigas from the three locations ranged between 2.64 and 3.15 g/100 g wet weight (Figure 1). The total lipid content of oysters significantly (p<0.05) varied across harvest seasons and farming locations. Within the year, it was the lowest in the months of May and September. The content of total lipids recovered from Ninh Hoa oysters was slightly higher than that for Cam Lam and Van Ninh, which can be related to better food availability and quality and environmental conditions in Ninh Hoa compared to the other two areas. Lipid accumulation and depletion in molluscs depend on the stage of gonad maturation, food quality and supply, and the influence of environmental conditions on metabolic activity [Su et al., 2006]. Lipids in oysters play an important role in gametogenesis and act as an energy source [De La Parra et al., 2005]. They are accumulated when food is available, and during gonad maturation when there is conversion of glycogen into lipids. The low lipid content in oysters harvested in May and September in our study corresponded to the spawning period, an energy-intensive process. Post spawning, oysters accumulate storage lipids, especially triacylglycerols, in the resting phase [Napolitano & Ackman, 1992; De La Parra et al., 2005]. Sterols are accumulated during gonad development in preparation for the reproductive processes [Pazos et al., 1996]. Phytoplankton blooms during the rainy season in November and December probably were responsible for the increased lipid content of oysters (Figure 1). Previous studies have indicated seasonal and locational variations in the lipid content of C. gigas [Dridi et al., 2007; Linehan et al., 1999; Pazos et al., 1996]. Linehan et al. [1999] observed that the highest total lipid content of C. gigas was in December (8.7% dry weight) and the lowest in September (7.8% dry weight). Dagorn et al. [2016] reported the maximum total lipid content of the invasive C. gigas in April (8.6% dry weight) and the minimum in January (7.1% dry weight). In a study by Asha et al. [2014] on Crassostrea madrasensis, the average total lipid content on a wet weight basis was 3.25%. In the present study, the range of total lipid content for the C. gigas from the three locations was higher than that reported by Martino & Cruz [2004] for mangrove oyster Crassostrea rhizophorae (1.5–2.0 g per 100 g of the wet weight) collected at the mangrove of “Barra de Guaratiba” district, Brazil. These differences may be due to the location, environmental conditions, species, and food availability.
Phospholipid content
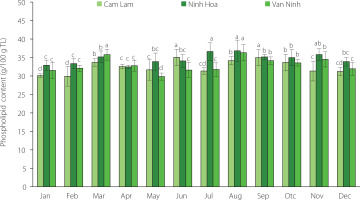
Phospholipids accounted for 29.84–36.82 g/100 g TL recovered from C. gigas and their content significantly (p<0.05) varied with oyster harvest season and farming location (Figure 2). The slightly higher phospholipid content was determined in the oysters from Ninh Hoa when compared to the other two locations (i.e., Cam Lam and Van Ninh). In general, the phospholipid content increased from January to March, started to decrease in April and May, then increased from June and peaked in August. High phospholipid contents in January to March and in August can indicate increased formation of germinal cells (in males) during spermatogenesis. Phospholipid content increases during gonadal maturation as they form part of the structural membrane lipids and as lipovitellins that accumulate oocytes as a reserve for the cellular dividing process following fertilization [De La Parra et al., 2005]. Phospholipid accumulation also increases during the period of maturity preceding spawning. Previous studies have indicated seasonal and locational variation in the phospholipid content of oysters [Dagorn et al., 2016; Liu et al., 2020; De La Parra et al., 2005; Saito & Marty, 2010]. A study by Dagorn et al. [2016] showed seasonal variation in the phospholipid content of Crassostrea gigas on the French Atlantic coast with 50.4% and 28.1% phospholipids (% of the total lipid) determined in winter and spring, respectively. Pogoda et al. [2013] found that the phospholipid content of the off-shore cultivated C. gigas ranged between 39.6–61.8% of the total lipid while that for the species Ostrea edulis was in the range of 42.6–55.5% of the total lipids. In a study by Liu et al. [2020], the phospholipid content of the oyster C. talienwhanensis from the Yellow Sea area was in the range of 11.46–52.12% of the total lipids. The highest phospholipid content in this study from the three areas was lower than the maximum reported in other studies [Dagorn et al., 2016; Liu et al., 2020; Pogoda et al., 2013]. Phospholipids are synthesized and accumulated as a response to low water salinity and freezing temperatures [Fokina et al., 2018]. This might explain the low phospholipid values in this study in comparison to other areas. However, the seasonal variation in the phospholipid content was minimal when compared to that reported in other studies [Dagorn et al., 2016; Liu et al., 2020; Pogoda et al., 2013].
Free fatty acid content
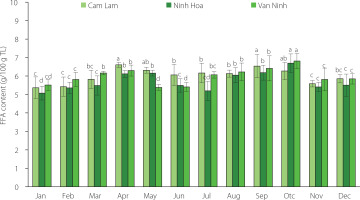
The free fatty acid (FFA) content of the lipids recovered from C. gigas for the three locations ranged between 5.07 and 6.70 g/100 g TL (Figure 3). At the p≥0.05, there were no significant differences in the FFA content of the lipids of oysters from the three farming areas across the harvest season. However, the FFA content of oysters from each farming location varied significantly (p<0.05) across the 12 months. The highest FFA content was observed in oysters harvested in April and October which corresponded to the two spawning seasons. Free fatty acids are generated after the degradation of the lipids by enzymes in dead cells [Saito & Marty, 2010]. They also represent an immediate energy source with their content being proportional to the metabolic demand [Viladrich et al., 2016]. Stress conditions like thermal stress and starvation can increase the FFA content. Increased FFA content has been reported in gorgonians after spawning as a mechanism to overcome reproductive stress [Viladrich et al., 2016]. The increase in FFA content of C. gigas recorded in our study in April was probably a way for the oysters to meet the metabolic demands for the spawning period as FFAs were obtained from lipid reserves. It could also be a mechanism to overcome thermal stress as temperatures increase. Several studies have also reported low FFA levels in oysters harvested from different areas. Pogoda et al. [2013] reported low levels of free fatty acids in Ostrea edulis and C. gigas of 0.5–2.8% and 0.4–3.5% of the total lipids, respectively. Similar to this study, the highest values of FFA were recorded in April. FFA content determined for C. talienwhanensis from the Yellow Sea area was in the range of 0.65–7.88% of the total lipids [Liu et al., 2020]. Saito & Marty [2010] reported high levels of FFA for the oyster C. gigas (2.7–8.1% of the total lipid).
Neutral and polar lipid contents
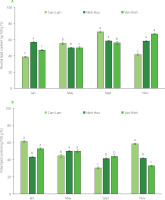
Total lipids recovered from C. gigas were fractionated into neutral lipids (38.75–69.68 g/100 g TL) and polar lipids (32.86– –61.25 g/100 g TL) using column chromatography (Figure 4). A clear correlation was found – the higher the neutral lipid content, the lower the polar lipid content and vice-versa. Neutral lipids constituted a major portion of the lipids in the oysters from the three locations. Their content was higher in the oysters harvested in September (56.19–69.68 g/100 g TL) from the three locations and lower in January (38.75–56.99 g/100 g TL). For oysters farmed in Cam Lam, the content of polar lipids recovered in January and November was higher than that of the neutral lipids. Also, in January, the content of polar lipids recovered from oysters in Van Ninh was higher compared to the neutral lipid content. There was an equal distribution between neutral and polar lipids recovered in May for the oysters farmed in Ninh Hoa and Van Ninh at 50 g/100 g TL. Neutral lipids recovered from Cam Lam oysters increased from January (38.75 g/100 g TL) to September (69.68 g/100 g TL) and then decreased in November (41.62 g/100 g TL). Polar lipid levels on the other hand decreased from January (61.25 g/100 g TL) to September (30.32 g/100 g TL) and then started to increase again in November (58.38 g/100 g TL). In Ninh Hoa oysters, the percentage of neutral and polar lipids was relatively stable across the four months. Polar lipids are structural components of the membranes, and their content varies little irrespective of the external parameters and nutritional changes [Soudant et al., 1999]. In turn, the neutral lipid content and composition reflect the diet of the oysters and are used as a biomarker in bivalves [Pernet et al., 2006]. The ratio of polar lipids to neutral lipids is an indicator of the nutritional condition of the organism with values ≤1 showing good nutritional status [Pogoda et al., 2013]. Triacylglycerols were the main constituent of neutral lipids in Mytilus spp. [Fokina et al., 2018], oyster Crassostrea rhizophorae [Liu et al., 2020], and oyster Crassostrea gigas [Saito & Marty, 2010], and it was found that they are part of the principal energy reserves in marine invertebrates. They are accumulated in periods of high food availability and depleted during food scarcity. The relative stability of neutral lipids in oysters harvested in Ninh Hoa, in our study, can be indicative of better food quality and availability at all times. Also, high levels of neutral lipids recovered in November in Van Ninh were probably due to phytoplanktonic blooms during the rainy season. Low levels of neutral lipids in January may suggest low food availability. Increased levels of neutral lipids recorded in May in oysters from the farming areas of Cam Lam and Van Ninh during the spawning season are probably due to the accumulation of lipids and conversion of glycogen into lipids during gametogenesis. In a study on C. gigas by Dagorn et al. [2016], neutral lipids accounted for 40% in winter (January) and 64.5% in spring (April). Similar to this study, low levels of neutral lipids were recorded in January. High levels of neutral lipids in this study were consistent with findings from previous studies [Dagorn et al., 2016; Pazos et al., 1996; Pogoda et al., 2013; Saito & Marty, 2010].
Fatty acid composition
The contents of fatty acids, total saturated fatty acids (SFAs), monounsaturated fatty acids (MUFAs), and polyunsaturated fatty acids (PUFAs) of muscle lipids of C. gigas cultured in the three farming locations are shown in Table 1. Lipids recovered from C. gigas were abundant in SFAs (30.89–39.16 g/100 g TL), followed by PUFAs (28.13–35.88 g/100 g TL), and MUFAs (19.32–23.75 g/100 g TL). The main PUFAs were the n-3 PUFA with eicosapentaenoic acid (EPA, 20:5n3, 9.09–13.77 g/100 g TL) and docosahexaenoic acid (DHA, 22:6n3, 6.71–16.47 g/100 g TL) being the dominant fatty acids from the three locations across the harvest season. The major MUFA in oyster muscle was oleic acid (18:1n9, 6.96–10.00 g/100 g TL) while the most abundant SFA was palmitic acid (16:0, 21.14–27.27 g/100 g TL). Palmitic acid, EPA, and DHA are major components of phospholipids and are typical of marine animal lipids [Pogoda et al., 2013]. Since EPA and DHA are membrane components, their contents vary slightly throughout the year. Previous studies have also indicated that oyster was abundant in EPA and DHA [Liu et al., 2020; Martino & Cruz, 2004; Saito & Marty, 2010]. EPA levels were slightly higher in this study than these of DHA in general, similar to a study by Pazos et al. [1996] in which the contents of EPA in triacylglycerols and phospholipids of C. gigas were higher than these of DHA. High EPA levels are a reflection of EPA-rich phytoplankton. EPA has also been shown to have an energetic role [Qin et al., 2021]. The arachidonic acid (C20:4n6) is a precursor of prostaglandins which influences reproduction in molluscs [Soudant et al., 1999]. The proportion of n-6 fatty acids, especially C20:4n6, indirectly shows depletion and accumulation of lipid reserves through an increase or decrease, respectively [Pogoda et al., 2013]. In this study, there was a locational variation in the content of C20:4n6 in the profile of oyster fatty acids (Table 1). Lipids recovered from the oysters cultivated in Ninh Hoa had a lower content of this fatty acid compared to the oysters from the other two locations. Low lipid levels in Ninh Hoa oysters can indicate better food availability and quality compared to the other two locations as lipids are accumulated as reserves. The mean PUFAs, SFAs, and MUFAs content of C. gigas was not significantly different (p≥0.05) across the harvest seasons and locations. The order of PUFAs content from the three locations within the year decreased in the following order: January (32.87–35.88 g/100 g TL), November (31.37– –33.53 g/100 g TL), September (29.88–32.39 g/100 g TL), and May (28.13–32.91 g/100 g TL). Studies have shown an inverse correlation between temperature and the amount of PUFA in oysters during the different months [Pazos et al., 1996]. The insignificant (p≥0.05) seasonal variation in the PUFA content of C. gigas in our study can be explained by the fact that the water temperature does not vary much throughout the season at three farming locations. Marine bivalves have been indicated to have a special requirement for n-3 PUFAs accumulating these rather than n-6 [Abad et al., 1995]. This explains the higher levels of n-3 PUFAs compared to n-6 PUFAs in this study.
In a study by Futagawa et al. [2011] on C. gigas from two farming areas in Japan, SFAs accounted for 41.0–44.2% of the total lipids while MUFAs and PUFAs accounted for 14.8–22.4% and 35.2–42.2%, respectively. Asha et al. [2014] reported that PUFAs constituted the majority of the fatty acid pool in C. madrasensis followed by SFAs and lastly MUFAs. Similarly, EPA and DHA were found to be the predominant PUFAs. In a study by Liu et al. [2020], PUFAs (39.04–48.77% of total lipids) were the dominant FAs followed by SFAs (26.96–3 7.09% of total lipids) and MUFAs (19.43–28.85% of total lipids) in C. talienwhanensis from the Yellow Sea area. PUFA content in the cited study was higher than that in our study; however, similar to our study, palmitic acid was the most abundant SFA and oleic acid was the dominant MUFA. EPA and DHA were also the most prevalent PUFAs. Pogoda et al. [2013] also reported that the FAs of Ostrea edulis and C. gigas were dominated by palmitic acid (C16:0), EPA, and DHA similar to our study. The fatty acid composition of oysters is influenced by intrinsic (e.g., sex, age, size) and extrinsic factors (e.g., temperature, salinity, and diet) [Martino & Cruz, 2004]. A decrease in temperature leads to increased unsaturation of fatty acids to ensure membrane fluidity. At higher temperatures, an increase in phospholipid content is necessary to counteract excess fluidity, which explains the increased SFAs content in the oyster [Martino & Cruz, 2004].
The C. gigas lipid quality, expressed as a thrombogenicity index (TI) and an n-3/n-6 ratio, varied within the harvest season and farming location (Table 1). The TI order was as follows: September (0.44–0.46), May (0.40–0.44), November (0.38–0.430), and January (0.30–0.42). TI is related to the risk of thrombosis and its high values (>1.0) are considered dangerous to human health [Chakraborty et al., 2016]. The values of TI in this study were less than 1. The second lipid quality parameter, n-3/n-6 ratio, ranged from 1.45 to 4.55, and lipids recovered from the oysters harvested in Ninh Hoa exhibited its higher values compared to the oysters from the other two farming locations (Table 1). The n-3/n-6 ratios in our study were slightly higher than those reported by Liu et al. [2020] for C. talienwhanensis; however, were similar to those published by Martino & Cruz [2004] for mangrove oyster Crassostrea rhizophorae. Generally, oysters from tropical areas have been reported to have lower n-3/n-6 ratios compared to those from temperate areas [Lira et al., 2013]. Linehan et al. [1999] found the n-3/n-6 ratio for C. gigas as 5.88 in winter and 4.35 in summer. n-3 Fatty acids are important in diets as they play a vital role in body function regulation [Swanson et al., 2012]. However, a high intake of n-6 is implicated in the increased incidence of inflammatory diseases as n-6 and n-3 fatty acids compete for metabolic enzymes [Bhardwaj et al., 2016]. Therefore, the consumption of marine organisms, like oysters, is important as they are rich in n-3 fatty acids.
Table 1
Fatty acid composition (g/100 g total lipid) and nutritional quality indexes of lipids of Crassostrea gigas oysters cultured in Khanh Hoa coast at three locations and harvested in different months.
CONCLUSIONS
The results of this study indicate that both the harvesting season and farming location significantly influenced the lipid profile of muscle of Crassostrea gigas cultured in Khanh Hoa province, Vietnam. Oysters harvested from the three farming areas exhibited variations in lipid content, with a higher lipid content determined in the oysters from Ninh Hoa. A decreased lipid content and increased free fatty acid content were found during the two spawning seasons (April and September). Neutral lipids constituted a larger proportion of the total lipids of oysters harvested from the three farming areas. Oysters from Ninh Hoa exhibited lower thrombogenicity index (TI) values and higher n-3/n-6 ratios compared to those from Cam Lam and Van Ninh, which indicates their higher nutritional value. Further studies that consider seasonal and locational variations in nutritional components, such as protein, glycogen, mineral content, etc., could be conducted to determine the optimal harvesting time.